New fluorescent organic nanoparticles to see the invisible
A new nanomaterial for bioimaging has been developed by researchers at NANBIOSIS Unit 6 Biomaterial Processing and Nanostructuring Unit from the Nanomol group from ICMAB-CSIC and CIBER-BBN . The researchars are also members of the TECNIO technology transfer network ACCIÓ-Generalitat de Catalunya, together with the New Jersey Institute of Technology (NJIT, USA) and the University of Parma (UNIPR, Italy). The results of the study are the result of the TECNIOspring PLUS project co-financed by ACCIÓ and the European Commission.
It is true that it is very difficult to understand what happens in our bodies if we are unable to visualise it. For example, we currently know that tumour cells have the capacity to grow without control thanks to various microscopic techniques that have allowed us to enlarge them to such an extent that we have been able to see each cell perfectly. The design of microscopes and the optical and electronic engineering behind them has advanced very rapidly in recent years. In fact, the 2014 Nobel Prize in Chemistry was awarded to researchers Eric Betzig, William E. Moerner and Stefan Hell, for the development of super-resolution fluorescence microscopy. These advances have made it possible to see even what is inside cells, reaching the nanometer scale with high resolution.
Now, what happens when we are not able to see what we are looking for? This is where fluorescent probes come into play, molecules that provide a signal: they emit light at a certain wavelength once they are excited. These probes must meet a series of requirements, among which are: they must have a high luminosity or brightness, be totally biocompatible, and have high photo-stability and high dispersibility in physiological media.
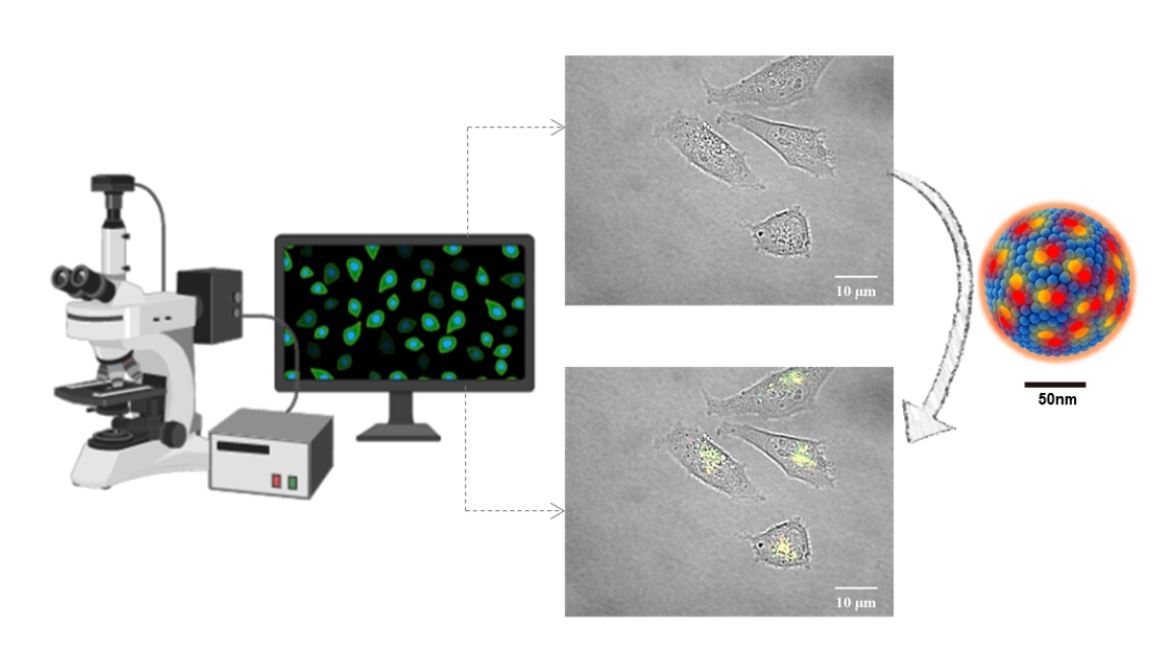
The Nanomol group has developed new fluorescent probes, specifically fluorescent organic nanoparticles (FONs). These new FONs are based on Quatsomes (QSs), nanovesicules produced by the same group through a green technology (Delos-susp, Nanomol Technologies SL), which are charged with fluorophores or fluorescent molecules – specifically two types of carbocyanins. The nanoparticles have an average diameter of 120 nm and have demonstrated good biocompatibility and high stability, both over time and once exposed to high power laser irradiation.
Characterization of nanovesicles was made at the ICTS “NANBIOSIS”, more specifically by the Unit 6 Biomaterial Processing and Nanostructuring Unit of CIBER-BBN.
“The brightness achieved is especially relevant: these new fluorescent nanoparticles are about 100 times brighter than other commercial fluorescent nanoparticles, such as Quantum Dots, thus allowing the acquisition of high quality images” explains Judit Morla-Folch, postdoctoral researcher of the Nanomol group at the ICMAB and first author of the study, published in the journal ACS Appl. Mater. Interfaces.
In addition, these nanoparticles have another singularity, and that is that they experience Förster resonance energy transfer, usually abbreviated as FRET. This phenomenon allows for improved image acquisition as it significantly reduces self-absorption and therefore background noise during bioimage acquisition. In addition, the FRET effect allows the integrity of the nanoparticle to be monitored, a great advantage for biomedical applications where it is necessary to know when the nanovesicle remains as a whole or it disintegrates.
In summary, the fluorescent organic nanoparticles (FONs) developed by the Nanomol group of the ICMAB-CSIC in collaboration with the NJIT (USA) and the UNIPR (Italy) constitute a promising platform for bioimaging and for the design of medical diagnostic kits.
Cover Figure: The new fluorescent organic nanoparticles allow to improve the visualization of cells and tissues under the microscope.
Reference article:
Dye-Loaded Quatsomes Exhibiting FRET as Nanoprobes for Bioimaging
Judit Morla-Folch, Guillem Vargas-Nadal, Tinghan Zhao, Cristina Sissa, Antonio Ardizzone, Siarhei Kurhuzenkau, Mariana Köber, Mehrun Uddin, Anna Painelli, Jaume Veciana, Kevin D. Belfield, and Nora Ventosa
ACS Appl. Mater. Interfaces 2020, 12, 18, 20253–20262
DOI: 10.1021/acsami.0c03040