U10-S8.
3D bio-impression of scaffolding for regenerative medicine
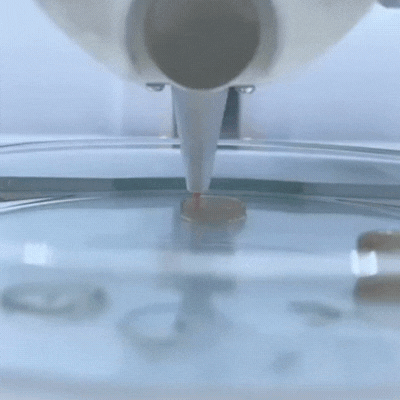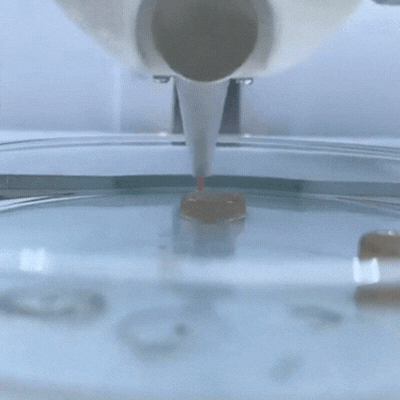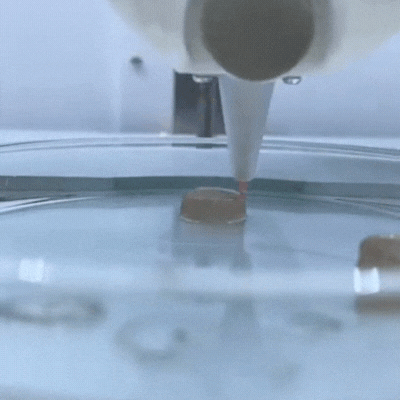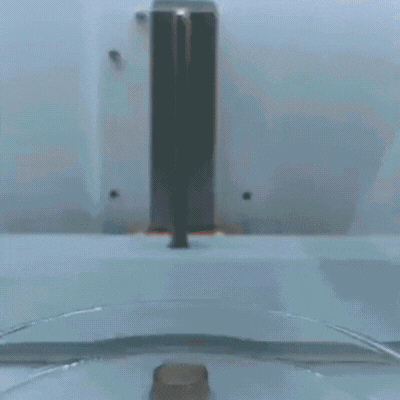
The principle of 3D bioprinting consists of selecting the most suitable biomaterials and cell types to prepare a Bioink that should be able to promote cell growth and differentiation and present appropriate mechanical properties of the target tissue.
This service possess a wide variety of 3D bioprinting techniques avaliable, such as extrusion, droplet, electrospining, electrowritting and stereolithography.
Customer benefits
One of the main characteristics of this additive manufacturing technique is its ability to bioprint the desired layers, with specific cell orientation , and desired morphology of the bioprinted 3D scaffold in order to ressemble, as much as possible, the tissue of interest. To achieve this goal, rheology, texturometry, printability and biological assays are carried out.
On the one hand, this technology can be employed to develop 3D scaffolds specific for the regeneration of particular tissues. On the other hand, this strategy offers a 3D environment that mimics the tissue/ organ of interest in order to test potential therapeutic tools, which goes in accordance with the implementation of the 3R principle (replace, reduce and refine).
Target customer
- Preclinical use for in vitro and in vivo models.
- Pharmaceutical industry (e.g. cosmetics)
References
- Lafuente-Merchan M, Ruiz-Alonso S, García-Villén F, Zabala A, de Retana AMO, Gallego I, Saenz-Del-Burgo L, Pedraz JL. 3D Bioprinted Hydroxyapatite or Graphene Oxide Containing Nanocellulose-Based Scaffolds for Bone Regeneration. Macromol Biosci. 2022 Nov;22(11):e2200236. doi: 10.1002/mabi.202200236.
- Lafuente-Merchan M, Ruiz-Alonso S, Zabala A, Gálvez-Martín P, Marchal JA, Vázquez-Lasa B, Gallego I, Saenz-Del-Burgo L, Pedraz JL. Chondroitin and Dermatan Sulfate Bioinks for 3D Bioprinting and Cartilage Regeneration. Macromol Biosci. 2022 Mar;22(3):e2100435. doi: 10.1002/mabi.202100435.
- Ruiz-Alonso S, Villate-Beitia I, Gallego I, Lafuente-Merchan M, Puras G, Saenz-Del-Burgo L, Pedraz JL. Current Insights Into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics. 2021 Feb 26;13(3):308. doi: 10.3390/pharmaceutics13030308.
Additional information
3D Bioprinters: BIO X 3D Bioprinter –CELLINK (left); R-GEN 100 –REGENHU (right).
Bioprinted 3D scaffold