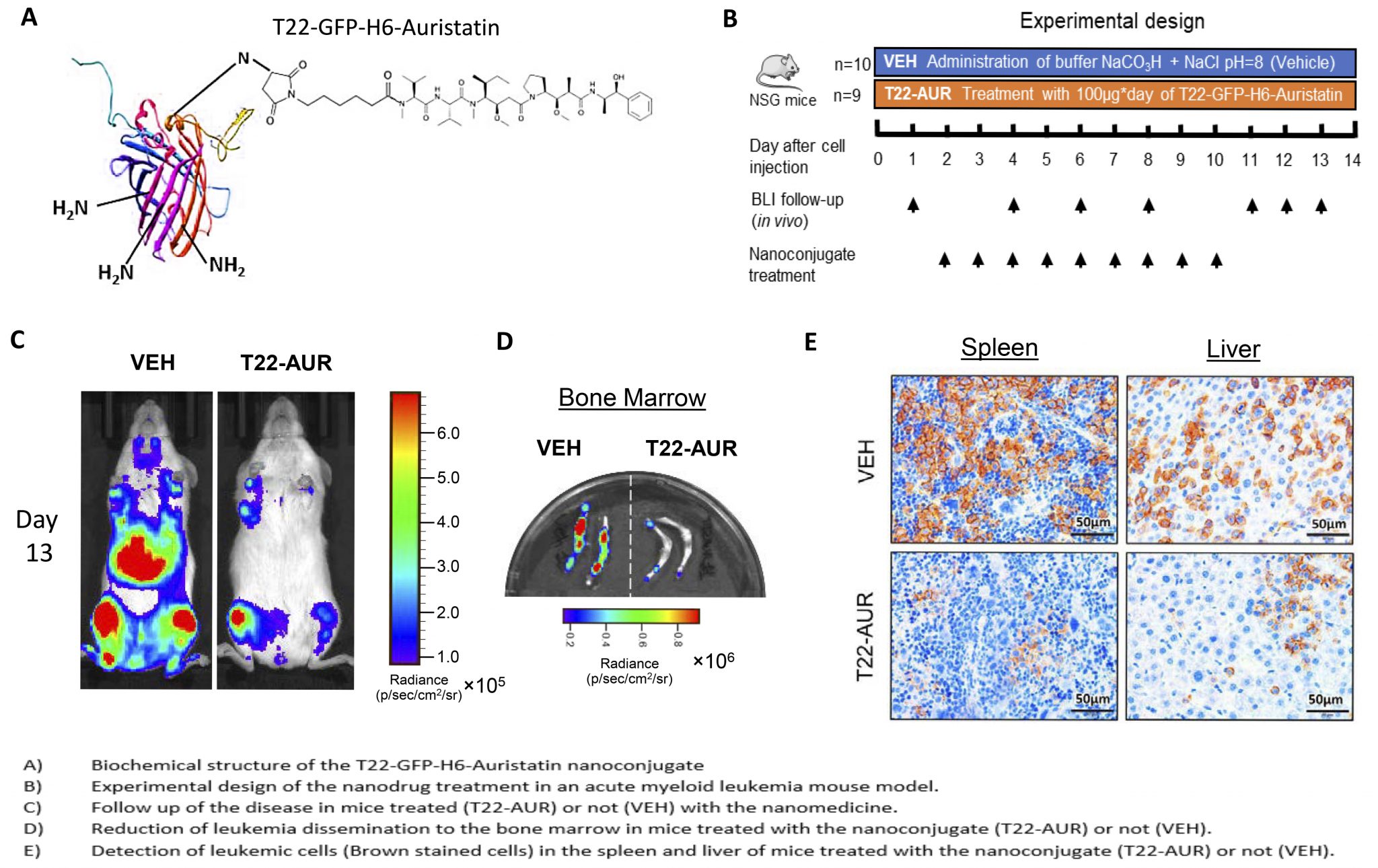
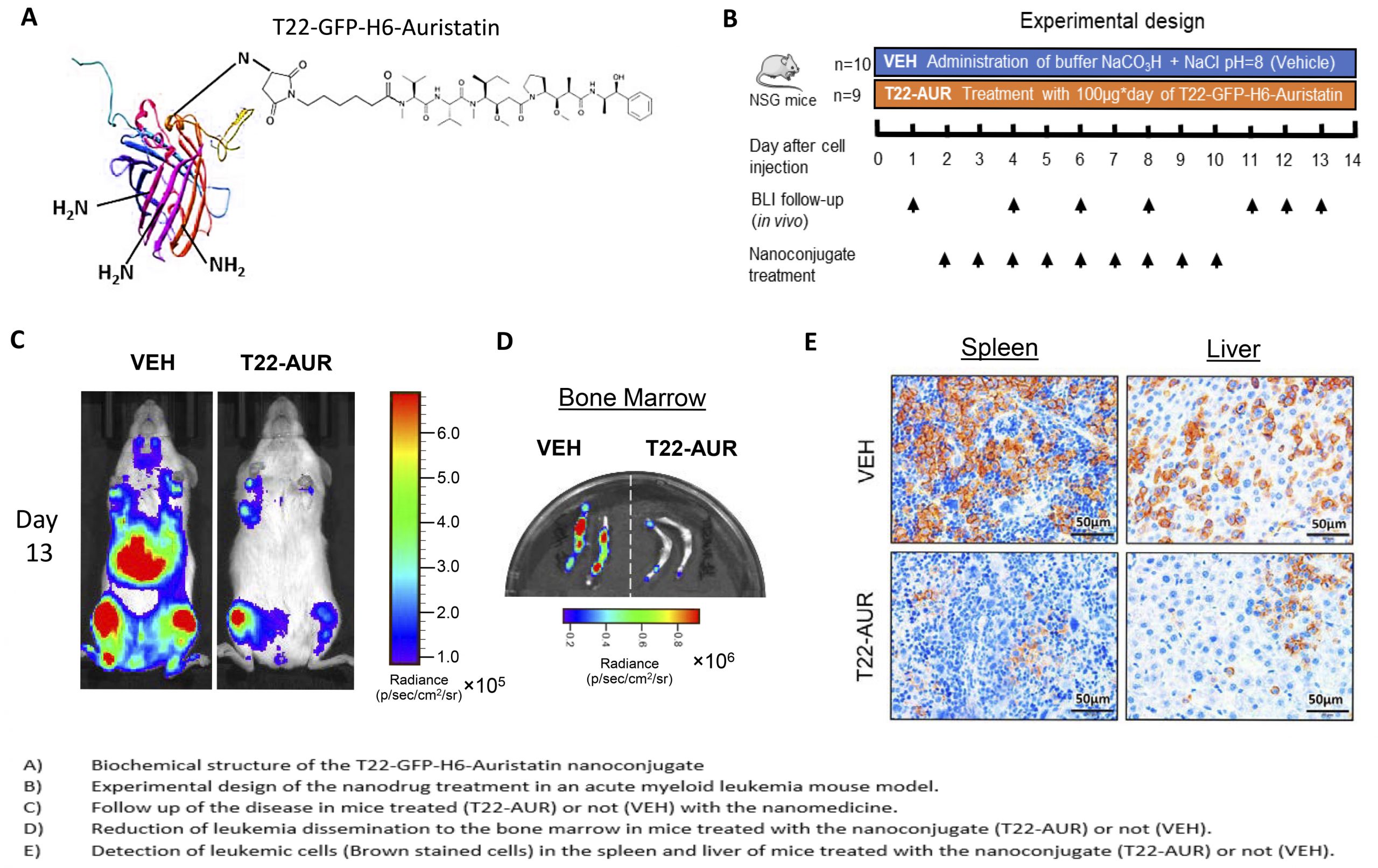
An Auristatin-based nanoconjugate reduces leukemia burden in a disseminated AML model
Researchers of the Nanotoxicology Unit of the the CIBER-BBN ICTS NANBIOSIS (u18-nanotoxicology-unit), leaded by Ramon Mangues and Isolda Casanova at the Research Institute of the Hospital de Sant Pau and of the NANBIOSIS (nanbiosis.es) Protein Platform (u1-protein-production-platform-ppp) leaded by Antonio Villaverde and Neus Ferrer Miralles of the Institute of Biotechnology and Biomedicine at the Autonomous University of Barcelona, have developed a novel protein-Auristatin nanoconjugate that specifically targets CXCR4-overexpressing acute myeloid leukemia (AML) cells. It selectively accumulates in target cancer cells expressing this receptor and deliver the toxin Auristatin within their cytosol. There, Auristatin potently blocks microtubule polymerization, provoking mitotic catastrophe, followed by apoptotic induction. Since Auristatin can kill both cycling and quiescent cells, the administration of the nanoconjugate at repeated dosage is able to dramatically reduce the leukemia burden in circulating blood, bone marrow, liver and spleen; thus, producing a potent antineoplastic effect, in the absence of systemic toxicity.
It is known that CXCR4 overexpression is involved in bopne marrow colonization by leukemic cells, displacing normal hematopoietic stem cells, an effect that associates with quiescence, resistance to classical chemotherapy, development of minimal residual disease and relapse, which leads to shorter patient survival. Therefore, this Auristatin-based nanoconjugate could be a novel approach for the treatment of CXCR4-overexpressing AML that relapses after classical chemotherapy, offering hope to an effective clinical translation and industrial transfer, aqn activity that which could increase the effectiveness of AML treatment while reducing the adverse effect associated with current therapy.
Reference:
Pallarès V, Unzueta U, Falgàs A, Sánchez-García L, Serna N, Gallardo A, Morris GA, Alba-Castellón L, Álamo P, Sierra J, Villaverde A, Vázquez E, Casanova I, Mangues R. An Auristatin nanoconjugate targeting CXCR4+ leukemic cells blocks acute myeloid leukemia dissemination. doi: 10.1186/s13045-020-00863-9.