Revolutionizing healthcare with recombinant proteins — unleashing safe, targeted drug delivery and tissue regeneration for groundbreaking therapeutic treatments.
Scientific Leaders of the project: Neus Ferrer Miralles, PhD.
Coordinator of the project: Mercedes Marquez, PhD.
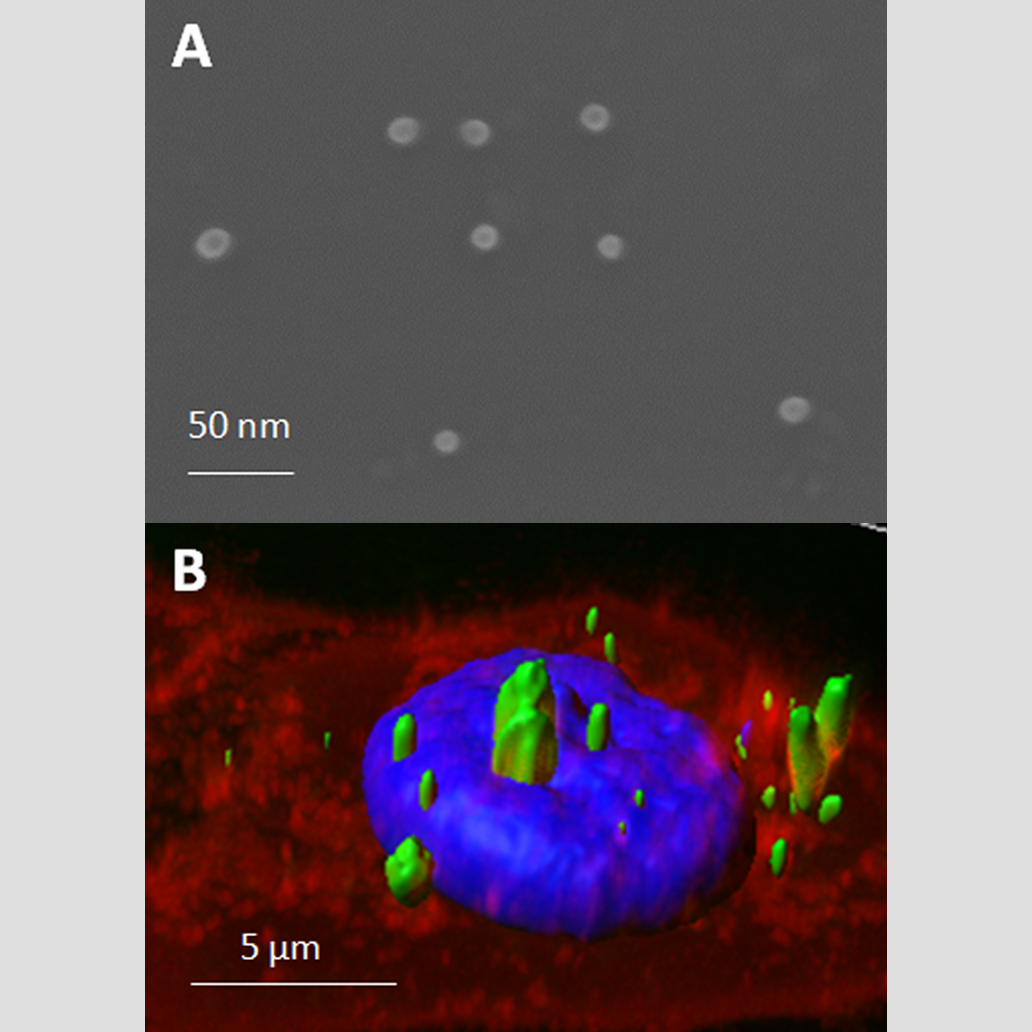
Advance in the life quality of patients is an essential purpose for biotechnology and nanomedicine. The development of highly tunable biomaterials suitable for therapeutic purposes perfectly matches the activity of the NANBIOSIS unit 1 PPP. In fact, recombinant proteins can be engineered to be used as effective, safe, non-invasive and cell selective nanomaterial in new therapeutic treatments. Protein-only nanostructures, either in soluble or Inclusion bodies form, have being successfully used both in human and animal health models, showing the highly potential and versatility of these nanomaterials.
Soluble protein nanoparticles
The use of recombinant proteins to form nanostructures have generated high expectations because their potential applicability, especially in targeted drug delivery. In this context, a novel tunable platform of multifunctional soluble proteins, engineered to spontaneously self-assemble into nanostructures, has been shown to be an excellent model for newly therapeutic approaches.
Engineered multifunctional proteins are generally easy to produce and purify allowing an easy and time saving screening of different protein building blocks for a wide range of applicability.
Services involved: Standar services
Projects are carried out essentially by U1 (PPP). Depending of the nature of the proteins, their purifications and characterization can be developed in collaboration with U2 (CAbS). Protein nanoparticles effectiveness and toxicity can be tested in collaboration of U18 (Nanotoxicology) and in vivo experiments are done by U20 (In Vivo Experimental Platform). These platforms are currently collaborating with U1 (PPP) on projects of this nature.
Some examples are described in the following publications:
 |
The PPP Unit 1 is at the Institute of Biotechnology and Biomedicine of the Autonomous University of Barcelona (IBB-UAB). It counts with the necessary facilities for the design, production, and purification of recombinant proteins. This facility is coordinated by the Nanobiotechnology Group, led by Prof. Antonio Villaverde. It has both highly specialized personnel and the necessary equipment to offer a “tailored” service for the design, production, and purification of recombinant proteins using both prokaryotic and eukaryotic expression systems. Its location allows this service to be linked with other complementary services available within the university (Cell Culture, Cytometry, Production of Antibodies, Microscopy, Proteomic, and Bioinformatic, X-ray crystallography, and Microarray and Sequencing Services), as well as facilitating the management of subsequent uses of the produced protein. |
 |
Unit 2 (CAbS) is managed by the scientific team of the Nb4D group (Nb4D), led bu M-Pilar Marcom with high expertise in hapten synthesis, conjugation and antibody production.We have an extensive track record of successful projects in developing polyclonal and monoclonal antibodies against small molecules and peptidesfor the development of diagnostic tools. These include, for example, the evaluation of carcinogenic processes, cardiovascular diseases, and infectious diseases. |
 |
Unit 18 (Nanotoxicology) is located in the Hospital de la Santa Creu i Sant Pau, in Barcelona, and is coordinated by Dr. Ramón Mangues, PI of the Oncogenesis and Antitumor Drug Group. The main objective of the Nanotoxicology Unit is to assess the toxicity of new drugs, nanoparticles or nanotechnology-based biomaterials in in vitro and in vivo systems, with the goal of optimizing lead compounds and identifying those with the highest probability of success in the preclinical programme due to their greater safety and tolerability or reduced toxicity. The Unit has rooms equipped for cell culture, for cryopreservation of samples and cell lines, and for sample preparation and analysis and animal facilities for in vivo experimentation. |
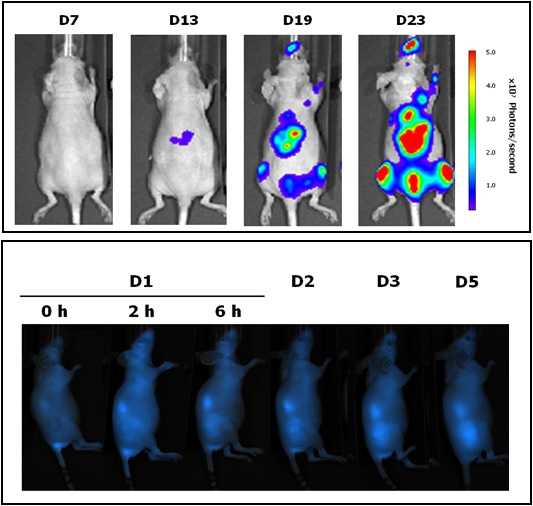 |
Located at the Valld’Hebron Research Institute (VHIR) in Barcelona, our In vivo Experimental Platform (U20) has three different sections, a Molecular Imaging section for in vivo, ex vivo and in vitro imaging studies (fluorescence, bioluminescence and X-rays), a preclinical animal model section and a preclinical histology section. All three sections are included within the Functional Validation & Preclinical Research (FVPR) of CIBBIM-Nanomedicine. This unit is lead by Dr. Ibane Abasolo (Head of FVPR) and supervised by the directorof CIBBIM-Nanomedicine, Dr. Simó Schwartz Jr. Its mission is to offer products and services for the preclinical proof-of-concept validation of therapeutic compounds and biomarkers with potential clinical applications. One of the distinguishing features of this unit is that basic preclinical studies including toxicology, histopathology and efficacy treatments are complemented with non-invasive optical imaging technologies. In vivo bioluminescence and fluorescence imaging allows monitoring of living mice longitudinally, offering real time insight into treatment efficacy, whole-body biodistribution and target mechanisms. Although in Spain there are other institutions with similar equipment, this is the only case in which imaging services can be combined with specifically devoted animal models. |