U20. Functional Validation & Preclinical Research (FVPR)
Order request
Description
Located at the Valld’Hebron Research Institute (VHIR) in Barcelona, U20 In vivo Experimental Platform has three different sections, a Molecular Imaging section for in vivo, ex vivo and in vitro imaging studies (fluorescence, bioluminescence and X-rays), a preclinical animal model section and a preclinical histology section. All three sections are included within the Functional Validation & Preclinical Research (FVPR) of CIBBIM-Nanomedicine.
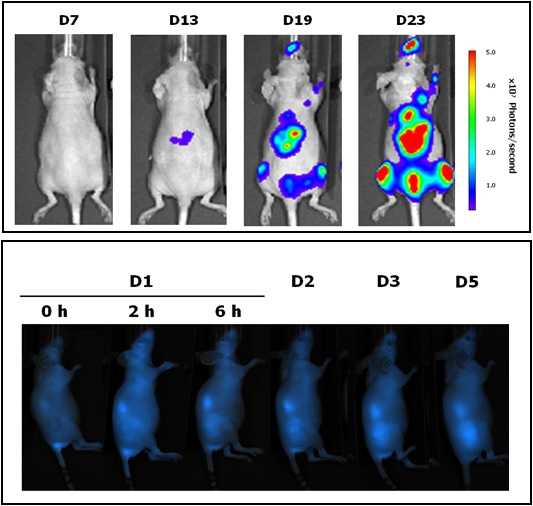
This unit is led by Dr. Ibane Abasolo (Head of FVPR). Its mission is to offer products and services for the preclinical proof-of-concept validation of therapeutic compounds and biomarkers with potential clinical applications. One of the distinguishing features of this unit is that basic preclinical studies including toxicology, histopathology and efficacy treatments are complemented with non-invasive optical imaging technologies. In vivo bioluminescence and fluorescence imaging allows monitoring of living mice longitudinally, offering real time insight into treatment efficacy, whole-body biodistribution and target mechanisms. Although in Spain there are other institutions with similar equipment, this is the only case in which imaging services can be combined with specifically devoted animal models.
Services
- U20-S01 Histological processing, section, staining and immunohistochemistry assays (Remote) OUTSTANDING
- U20-S02. Preclinical Experimental consultancy (Remote) OUTSTANDING
- U20-S03. Detection of microbial (bacterial and mycoplasma) or endotoxin contamination
- U20-S04. Hemocompatibility assays: haemolysis, platelet aggregation, plasma coagulation times and complement activation
- U20-S05. In vitro Efficacy assays of drugs, nanomedicines, biomaterials and others
- U20-S06. Non-invasive optical imaging (bioluminescence and fluorescence) (Remote) OUTSTANDING
- U20-S07. Animal models in oncology (Orthotopic, subcutaneous, intrasplenic and intravenous cell inoculation in syngeneic and immunodeficient mice (Remote) OUTSTANDING
- U20-S08. In vivo Efficacy Assays of drugs, nanomedicines, biomaterials and others (Remote) OUTSTANDING
- U20-S09. In vivo toxicology (Onsite&Remote) OUTSTANDING
- U20-S10. In vivo ADME and biodistribution assays (Remote) OUTSTANDING
- U20-S11. In vivo PK/PD assays (Remote) OUTSTANDING
- U20-S12. Immunotoxicity assays (On-site&Remote) OUTSTANDING
- U20-S13. X-ray imaging in vitro and in vivo (Remote) OUTSTANDING
FOR THOSE SERVICES IDENTIFIED AS OUTSTANDING, AT LEAST 20% OF THEIR CAPACITY IS OPEN UNDER COMPETITIVE ACCESS. SEE ANNEX 1 OF ACCESS PROTOCOL FOR DETAILS ON % OF OPENNESS FOR EACH SERVICE
U20. Services & Rates
Other projects
| Ref |
Title |
Funding Organism |
Unit Role |
| RTC-2014-2207-1 |
TERARMET: Development of therapies for the treatment of rare congenital metabolic diseases |
MINECO |
Participant |
Publications
2016
- Giannotti M.I., Abasolo I., Oliva M., Andrade F., Garcia-Aranda N., Melgarejo M. et al. Highly Versatile Polyelectrolyte Complexes for Improving the Enzyme Replacement Therapy of Lysosomal Storage Disorders. ACS Applied Materials and Interfaces. 2016;8(39):25741-25752.
- Pesarrodona M., Fernandez Y., Foradada L., Sanchez-Chardi A., Conchillo-Sole O., Unzueta U. et al. Conformational and functional variants of CD44-targeted protein nanoparticles bio-produced in bacteria. Biofabrication. 2016;8(2):-.
- Cabrera I., Abasolo I., Corchero J.L., Elizondo E., Gil P.R., Moreno E. et al. α-Galactosidase-A Loaded-Nanoliposomes with Enhanced Enzymatic Activity and Intracellular Penetration. Advanced Healthcare Materials. 2016;5(7):829-840.
- Cespedes M.V., Fernandez Y., Unzueta U., Mendoza R., Seras-Franzoso J., Sanchez-Chardi A. et al. Bacterial mimetics of endocrine secretory granules as immobilized in vivo depots for functional protein drugs. Scientific Reports. 2016;6:-.
News U20
23 May

“Fabry Connections” united patients, researchers, and clinicians at ICMAB to advance Fabry disease care and nanomedicine-based therapies. Barcelona, april 2025. On Tuesday, 29 April 2025, the Institute of Materials Science of Barcelona (ICMAB, CSIC) hosted the event “Fabry Connections: Science, Patients, and Future”, bringing together around 50 participants including researchers, clinicians, patients, and patient association representatives. The event aimed to create a multidisciplinary platform for sharing knowledge and advancing the future of Fabry disease research and treatment. Attendees at the Fabry Connections event at ICMAB | ICMAB-CSIC As we announced back in february 28, this special event was held within Fabry Diseas[...]
04 Feb
Vall d’Hebron develops magnetic nanoparticle hyperthermia to enhance pancreatic cancer treatment, now advancing to clinical trials. Barcelona, january 2025. A clinical trial targeting patients with locally advanced pancreatic cancer has been approved following a study led by the Vall d’Hebron Research Institute (VHIR). A preclinical study led by the Clinical Biochemistry, Drug Targeting, and Therapy (CB-DDT) group at Vall d’Hebron Research Institute (VHIR), which Unit 20 of NANBIOSIS is integrated, has proposed the use of magnetic nanoparticles and hyperthermia to enhance the treatment of pancreatic adenocarcinoma. The goal is to penetrate the desmoplastic stroma—the dense tissue surrounding these tumors—which acts[...]
29 Jan

Fabry disease therapy nanoGLA, developed by NANBIOSIS and our partners, shows superior efficacy in preclinical trials, targeting systemic and brain symptoms. Barcelona, january 2025. An international research team led by the Institute of Materials Science of Barcelona (ICMAB-CSIC) and CIBER-BBN, in collaboration with the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC), has developed a groundbreaking nanotechnology-based therapy called nanoGLA for the treatment of Fabry disease. The innovative solution has shown remarkable efficacy in preclinical studies and has been published in the open-access journal Science Advances (see below for reference links). What is Fabry disease? Fabry disease is a rare genetic[...]
25 Nov
Symposium on nanomedicine vs cancer: experts gather Nov 28 in Málaga to tackle tumor-targeting challenges and advance innovative therapies. Málaga, november 2024. On November 28th at 9:00 AM, the Salón de Actos at IBIMA (C/Severo Ochoa, 35, Málaga) will host the symposium “Targeting in Nanomedicine Against Cancer,” bringing together leading experts to discuss the critical challenges and advances in applying nanomedicine to oncology. Organized by IBIMA, this event will shed light on one of the most pressing issues in cancer nanomedicine: the difficulty of delivering nanostructures to tumor cells. The challenges to overcome cancer Cancer remains one of the world’s[...]
09 Oct
Directors of Unit 20 and Unit 3, Ibane Abasolo and Miriam Royo, show us the ReachGlio project, which uses nanomedicines to slow glioblastoma growth by targeting tumors in the brain, improving drug delivery through nanoparticles. Barcelona, october 2024. Each year, on October 9th, Nanotechnology Day is celebrated, a discipline dedicated to understanding and utilizing matter at a nanometric scale for purposes such as industrial or medical applications. Nanotechnology plays a fundamental role in many research lines developed at the Institute for Advanced Chemistry of Catalonia (IQAC-CSIC) and the Vall d’Hebron Research Institute (VHIR). “Our goal is to propose one or more clinical trials in[...]
16 May

A new project with the participation of NANBIOSIS Unit 20 pioneers thermosensitive hydrogels for localized ovarian cancer treatment, minimizing side effects and enhancing efficacy. May 2024, VHIR/FVPR/CIBER-BBN (Barcelona). As the world recently commemorated Ovarian Cancer Day, from NANBIOSIS we proudly highlight the groundbreaking work of our collaborators in the fight against this deadly disease. Ovarian cancer stands as the seventh leading cause of cancer-related deaths, with Europe bearing the brunt of its impact, recording over 44,000 fatalities annually. In a bid to revolutionize treatment paradigms and enhance patient outcomes, researchers at the Clinical Biochemistry, Drug Delivery and Therapy (CB-DDT) Group,[...]
01 Mar

NANBIOSIS researchers pioneer novel treatments for Lysosomal Storage Diseases utilizing extracellular vesicles and liposomes, offering hope to patients. 1 March 2024, Vall d’Hebron Research Institute/ICMAB-CSIC (Barcelona) Lysosomal Storage Diseases (LSDs) encompass a group of rare disorders caused by mutations in lysosomal proteins. These mutations can lead to dysfunctional proteins responsible for breaking down cellular materials, resulting in the accumulation of deposits. Such accumulations can manifest in various neurological symptoms, ranging from progressive neurodegeneration to severe cognitive impairment. Often emerging in childhood, LSDs tragically culminate in premature death for many patients. Currently, up to 14 subtypes of LSDs can be treated[...]
27 Feb

For Rare Disease Day, we raise awareness through 2 events: 1 in Barcelona (Feb 28) & another in Madrid (Feb 29). Collaboration & research in focus. 28-29 February 2024, Rare Disease Day 2024 NANBIOSIS is actively involved in promoting awareness and understanding of rare diseases, Due to their lower incidence, these conditions are often face neglect in medical research and industrial treatment. To spotlight this crucial issue, NANBIOSIS is pleased to announce its participation in two significant events coinciding with Rare Disease Day celebrations. These events will address various aspects of rare and minority diseases research and treatment. Event 1:[...]