
Fast-track your nanomedicine’s path to market with NANBIOSIS: tailored preclinical studies, cutting-edge imaging, and comprehensive analytics, all while ensuring ethical compliance and regulatory adherence.
Scientific leaders of the project: Francisco Miguel Sánchez-Margallo, Verónica Crisóstomo, Beatriz Moreno, José Luis Pedraz, Salvador Gil & Ramón Mangues.
Coordinator of the project: Ibane Abasolo & Francisco Miguel Sánchez-Margallo.
One of the causes that increase the time to market of nanotherapeutics is its preclinical validation. The standard protocols for more traditional drugs are not applicable for nanomedicines. NANBIOSIS offers a tailored preclinical study plan to meet the demands of each nanomedicine product. This is developed in collaboration with the user and starting from a set of basic analytical tests to more sophisticated experiments, in a variety of animal models. In addition, our experiments can be run either under GLP or non-GLP conditions, deppending on the stage of your project.
Studies of nanomedicines are conducted in animals utilizing a variety of models and state of the art imaging techniques (fluorescence, bioluminescence, TAC, MRI, CT, Echography, etc.). This is complemented with a wide variety of analysis, including clinal tests: hematology, biochemistry, coagulation, urinalysis, tumor markers, hormones, fertility studies, cardiac markers, metabolic study, etc. NANBIOSIS collaborates with you in the selection of the most suitable animal species, and we can develop experimental pathologies in different models (large white pig, sheep, goat, beagle dog, minipig, rabbit, rat and mouse) upon request. Needless to say, all these experiments follow strictly the most ethical approaches and comply with the EU regulations on animal research.
Some examples are described in the following publications
In the development of this biomedical solution, the following services are involved:
Efficacy studies of nanomaterial are conducted in animal utilizing a variety of models. We collaborate in the selection of the most suitable animal species and can developexperimental pathology in different animal models (large white pig, sheep, goat, beagle dog, minipig, rabbit, rat and mouse) upon request. At present we have some models of pathologies available:
| Patology | Animal Model | Unit | References | |
| Skin | Wounds/skin regeneration | Rat | U10 | |
| Skin ulcers | Diabeticpig, mouse | U19, U21, U22, U24 | ||
| Diabeticulcers in cornea | Rabitt | U19,U21, U22, U24 | ||
| Any ulcer model | Pig, mouse | U19,U21, U22 U24 | ||
| Eye | Ocular pathologies | Rabbit | U19,U21, U22 U24 | |
| Diabetes | Diabetes (Type II) | Pig | U19,U21, U22 U24 | |
| Skin ulcers | Diabetic pig, Rabit | U19,U21, U22 U24 | ||
| Diabetic ulcers in cornea | Rabitt | U19,U21, U22 U24 | ||
| Bone | Bone regeneration: calotte & hip | Rat | U10 | |
| Brain | Alzheimer | Mouse | U10 | |
| Parkinson | Rat | U10 | ||
| Cerebral ischemia | Rat | U19,U21, U22 U24 | ||
| Heart/Vascular | Myocardial Infarction | Pig | U19,U21, U22 U24 | Crisostomo et al 2015 and 2017 |
| Vascular stenosis | Pig | U19,U21, U22 U24 | ||
| Arterial embolization | Pig | U19,U21, U22 U24 | ||
| Aneurysms | Pig | U19,U21, U22 U24 | ||
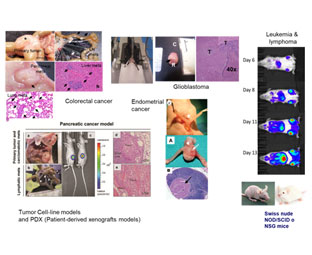
| Cancer | Colorectal* | Immunodeficient mouse. Orthotopic, subcucaneous, intrasplenic. | U18 & U20 | Botella et al., 2011; Fernández et al., 2015 |
| Colorectal | Immunocompetent (ApcMin) | Mateo-Lozano et al., 2017 | ||
| Pancreas* | Immuno deficient mouse. Subcutaneous. | U18 & U20 | Abasolo et al., 2009; Pujal et al., 2009 | |
| Endometrium | Immuno deficient mouse. | U18 | ||
| Glioblastoma* | Immunodeficient mouse. Orthotopic (steroatactic), subcucaneous. | U18 & U20 | Candiota et al., 2014 | |
| Lymphoma* | Immuno deficient mouse. | U18 | ||
| Leukemia* | Immuno deficient mouse. | U18 | ||
| Prostate* | Immuno deficient mouse. Orthotopic, subcucaneous. | U20 | Mateo et al., 2014 | |
| Patient-derived tumor graft (PDX) models (Endometrium, colorectal) | Immuno deficient mouse. | U18 | ||
| Breast (breast & Breast ductal carcinoma, adenocarcinoma & ductal adenocarcinoma,)* | Orthotopic (Intramamary fat pad). | U20 | Gener et al., 2015 | |
| Cervix | Subcutaneous. | U20 | Edinger et al., 1999 | |
| Lung | Subcutaneous. | U20 | Jakubowska et al., 2013 | |
| Ewing sarcoma* | Subcutaneous. | U20 | Ramon et al., 2013 | |
| Skin: Melanoma* | Subcutaneous & intravenous. | U20 | Adiseshaiah et al., 2014 | |
| Glioblastoma | Immuno competent mouse | U25 | Ferrer-Font L, et al. 2017 | |
| Pseudotumors | Pig | U19, U21, U22 U24 | ||
| Inflammation | Inflammation | Rat and mice (by LPS) | U20 | Thomas, Bath, Stover, Lambert, & Thompson, 2014 |
| Digestive | Hernias | Pig | U19, U21, U22 U24 | |
| Colecystitis | Pig | U19, U21, U22 U24 | ||
| Genitourinary | Urethral obstruction and stenosis/ endothelium inflammation | Pig | U19, U21, U22 U24 | |
| Unilateral and bilateral cryptorchidism | Dog | U19, U21, U22 U24 | ||
| Benign prostatic hiperplasia | Dog | U19, U21, U22 U24 | ||
| Endometryosis | Sheep | U19, U21, U22 U24 | ||
| Lysosomal storage disorders | Fabry disease | Immunocompetent mouse, KO for GLA gene | U20 | Cabrera et al., 2016 |
*Marked (luciferase, GFP, others)
REFERENCES
Abasolo, I., Pujal, J., Rabanal, R. M., Serafin, A., Navarro, P., Millán, O., & Real, F. X. (2009). FDG PET imaging of Ela1-myc mice reveals major biological differences between pancreatic acinar and ductal tumours. European Journal of Nuclear Medicine and Molecular Imaging, 36(7), 1156–1166.
Adiseshaiah, P. P., Patel, N. L., Ileva, L. V, Kalen, J. D., Haines, D. C., & McNeil, S. E. (2014). Longitudinal imaging of cancer cell metastases in two preclinical models: a correlation of noninvasive imaging to histopathology. Int J Mol Imaging. https://doi.org/10.1155/2014/102702
Botella, P., Abasolo, I., Fernández, Y., Muniesa, C., Miranda, S., Quesada, M., … Corma, A. (2011). Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation. Journal of Controlled Release, 156(2), 246–257. https://doi.org/10.1016/j.jconrel.2011.06.039
Cabrera, I., Abasolo, I., Corchero, J. L., Elizondo, E., Gil, P. R., Moreno, E., … Veciana, J. (2016). α-Galactosidase-A Loaded-Nanoliposomes with Enhanced Enzymatic Activity and Intracellular Penetration. Advanced Healthcare Materials, 5(7), 829–840. https://doi.org/10.1002/adhm.201500746
Crisóstomo V, Sun F, Maynar M, Báez-Díaz C, Blanco V, Garcia-Lindo M, Usón-Gargallo J, Sánchez-Margallo FM.Common swine models of cardiovascular disease for research and training. Lab Anim (NY). 2016 Feb;45(2):67-74. doi: 10.1038/laban.935.
Crisostomo V, Baez-Diaz C, Maestre J, Garcia-Lindo M, Sun F, Casado JG, Blazquez R, Abad JL, Palacios I, Rodriguez-Borlado L, Sanchez-MargalloDelayed administration of allogeneic cardiac stem cell therapy for acute myocardial infarction could ameliorate adverse remodeling: experimental study in swine. FM. J TranslMed. 2015 May 12;13:156. doi: 10.1186/s12967-015-0512-2.
Edinger, M., Sweeney, T. J., Tucker, A. A., Olomu, A. B., Negrin, R. S., & Contag, C. H. (1999). Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. https://doi.org/http://dx.doi.org/10.1016/S0959-8049(02)00410-0
Ferrer-Font L, Arias-Ramos N, Lope-Piedrafita S, Julià-Sapé M, PumarolaM,Arús C, Candiota AP. Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide. NMR Biomed. 2017Sep;30(9). doi: 10.1002/nbm.3748. Epub 2017 Jun 1. PubMed PMID: 28570014.
Fernández, Y., Forarada, L., García-Aranda, N., Mancilla, S., Suárez-López, L., Céspedes, M. V., … Abasolo, I. (2015). Bioluminescent Imaging of Animal Models for Human Colorectal Cancer Tumor Growth and Metastatic Dissemination to Clinically Significant Sites. Journal of Molecular Biology and Molecular Imaging, 2(2), 1019–33.
Gener, P., Gouveia, L. P., Sabat, G. R., de Sousa Rafael, D. F., Fort, N. B., Arranja, A., … Schwartz, S. (2015). Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine: Nanotechnology, Biology, and Medicine, 11(8), 1883–1892. https://doi.org/10.1016/j.nano.2015.07.009
Jakubowska, M., Śniegocka, M., Podgórska, E., Michalczyk-Wetula, D., Urbanska, K., Susz, A., … Plonka, P. M. (2013). Pulmonary metastases of the A549-derived lung adenocarcinoma tumors growing in nude mice. A multiple case study. Acta Biochimica Polonica. https://doi.org/10.1017/CBO9781107415324.004
Mateo-Lozano, S., Bazzocco, S., Rodrigues, P., Mazzolini, R., Andretta, E., Dopeso, H., … Arango, D. (2017). Loss of the EPH receptor B6 contributes to colorectal cancer metastasis. Scientific Reports, 7, 43702.
https://doi.org/10.1038/srep43702
Mateo, F., Meca-Cortés, Ó., Celià-Terrassa, T., Fernández, Y., Abasolo, I., Sánchez-Cid, L., … Thomson, T. M. (2014). SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Molecular Cancer, 13(1), 237. https://doi.org/10.1186/1476-4598-13-237
Pujal, J., Huch, M., José, A., Abasolo, I., Rodolosse, A., Duch, A., … Real, F. X. (2009). Keratin 7 promoter selectively targets transgene expression to normal and neoplastic pancreatic ductal cells in vitro and in vivo. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 23(5), 1366–1375.
Ramon, A. L., Bertrand, J. R., De Martimprey, H., Bernard, G., Ponchel, G., Malvy, C., & Vauthier, C. (2013). SiRNA associated with immunonanoparticles directed against cd99 antigen improves gene expression inhibition in vivo in Ewing’s sarcoma. Journal of Molecular Recognition. https://doi.org/10.1002/jmr.2276
Thomas, R. C., Bath, M. F., Stover, C. M., Lambert, D. G., & Thompson, J. P. (2014). Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides. https://doi.org/10.1016/j.peptides.2014.08.009
Molina I, Salvador F, Sánchez-Montalvá A, Artaza MA, Moreno R, Perin L, Esquisabel A, Pinto L, Pedraz JL. Pharmacokinetics of Benznidazole in Healthy Volunteers and Implications in Future Clinical Trials.Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01912-16. doi: 10.1128/AAC.01912-16. Print 2017 Apr.
| Single-Dose Acute Toxicity and Repeat-Dose Acute Toxicity Studies of anticancer nanomedicines in small animals | U18 U20 | Rafael et al., 2017 |
| Histopathology in small animals | U18 | Candiota et al., 2014 |
| Single-Dose Acute Toxicity and Repeat-Dose Acute Toxicity Studies of nanomedicines in large animals | U19, U21, U22 U24 | |
| Histopathology in large animals | U19, U21, U22 U24 | |
| Detection immune response: detection of Antibodies (anti PEG and others) | U2 | |
| PK of therapeutic antibodies | U2 |
References
Candiota, A., Acosta, M., Simões, R., Delgado-Goñi, T., Lope-Piedrafita, S., Irure, A., … Arús, C. (2014). A new ex vivo method to evaluate the performance of candidate MRI contrast agents: a proof-of-concept study. Journal of Nanobiotechnology, 12(1), 12. https://doi.org/10.1186/1477-3155-12-12
Rafael, D., Martínez, F., Andrade, F., Seras-Franzoso, J., Garcia-Aranda, N., Gener, P., … Schwartz, S. (2017). Efficient EFGR mediated siRNA delivery to breast cancer cells by Cetuximab functionalized Pluronic® F127/Gelatin. Chemical Engineering Journal. https://doi.org/10.1016/J.CEJ.2017.12.114
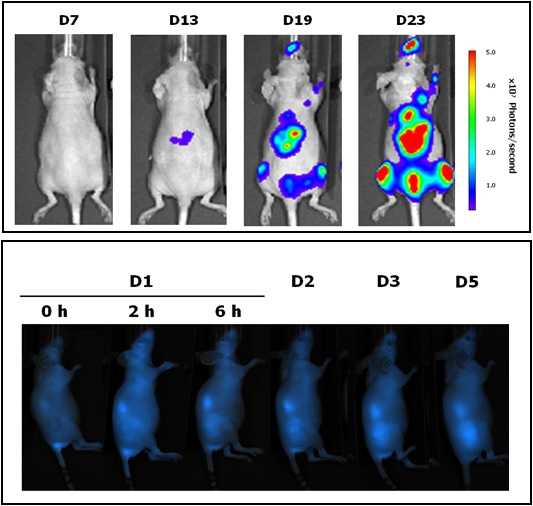
Mouse models for experimental oncology.
Nasal cavity.
1.5T MRI.
 |
CAbS (U2) is managed by the scientific team of the Nb4D group (Nb4D) with high expertise in hapten synthesis, conjugation and antibody production.We have an extensive track record of successful projects in developing polyclonal and monoclonal antibodies against small molecules and peptidesfor the development of diagnostic tools. These include, for example, the evaluation of carcinogenic processes, cardiovascular diseases, and infectious diseases. |
 |
Located at the Faculty of Pharmacy, University of the Basque Country (UPV/EHU), Campus of Alava in Vitoria. Our Unit 10 is led by Prof. José Luis Pedraz and consists of large laboratories for cell culture, chromatography equipment, sample preparation and characterization, and one specific for scale preparation of pharmaceutical formulations. Recently the group has incorporated 3D bioprinters of the last generation with different technologies based on extrusion, inkjet, among others. It has also incorporated self-assembly equipment of nanoparticles based on microfluidic technologies.
This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation. The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products. The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process. One of the singularities of this Units is that is GLP certified by the Spanish Medicament Agency |
 |
Unit 18 (Nanotoxicology) is located in the Hospital de la Santa Creu i Sant Pau, in Barcelona, and is coordinated by Dr. Ramón Mangues, PI of the Oncogenesis and Antitumor Drug Group. The main objective of the Nanotoxicology Unit is to assess the toxicity of new drugs, nanoparticles or nanotechnology-based biomaterials in in vitro and in vivo systems, with the goal of optimizing lead compounds and identifying those with the highest probability of success in the preclinical programme due to their greater safety and tolerability or reduced toxicity. The Unit has rooms equipped for cell culture, for cryopreservation of samples and cell lines, and for sample preparation and analysis and animal facilities for in vivo experimentation.
Equipment: The Unit has access to flow cytometers, sorters, confocal microscope and other equipment of the Platforms available at the Hospital Research Institute as well as housing facilities for small rodents (rat and mouse). |
 |
The Unit 19 is responsible for conducting regulatory preclinical studies for the pharmaceutical industry and interested companies. Among the studies that can be carried out are: in vivo toxicity studies, local and systemic tolerance and efficacy studies. Pharmacokinetic studies, dosage studies of test products and biocompatibility studies of new drugs and medical devices are also performed. All these safety studies are carried out using small and large animal models for the different organic systems, also including animal models of different pathologies.This Unit has a clinical analysis laboratory that performs a wide variety of analysis: (biochemistry, hematology, coagulation, urine analysis, blood gas analysis, different biomarkers, hormones, allergens) in various animal species (rat, mouse, rabbit, pig, sheep, dog, and cat). Specific methodologies are used in each animal species, having all techniques validated according to their physiological and pathological identity. This Unit has a clinical analysis laboratory that performs a wide variety of analysis (biochemistry, hematology, coagulation, urine analysis, blood gas analysis, different biomarkers, hormones, allergens) in various animal species, such as rat, mouse, rabbit, pig, sheep, dog, and cat. It also has a formulation laboratory equipped to formulate, reconstitute and guarantee the correct storage of the test products used in these studies. Both laboratories are certified with ISO-9001 and Good Laboratory Practices (GLP), strict quality standards that allow the production of high-precision results. |
 |
Located at the Valld’Hebron Research Institute (VHIR) in Barcelona, our In vivo Experimental Platform (U20) has three different sections, a Molecular Imaging section for in vivo, ex vivo and in vitro imaging studies (fluorescence, bioluminescence and X-rays), a preclinical animal model section and a preclinical histology section. All three sections are included within the Functional Validation & Preclinical Research (FVPR) of CIBBIM-Nanomedicine.
This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation. The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products. The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process. One of the singularities of this Units is that is GLP certified by the Spanish Medicament Agency |
 |
Our Unit 21 is equipped with experimental operating rooms with high technology and high field instrumentation to promote methodological and translational research class in Biomedicine, both minimally invasive surgery and new procedures and in preclinical development for technical evaluation, medical devices, biomaterials, etc. Surgical facilities allow the development and use of small and large animal models for research and experimental surgery in several longitudinal areas: cardiovascular diseases, liver and digestive diseases, respiratory and systemic diseases, endocrinology and urology, gynecological and reproductive diseases, pediatric diseases, orthopedics and traumatology, ophthalmology and experimental surgery in all organ systems have the infrastructure for transplant development, implants, prosthesis, biomaterials, etc. under high quality experimental conditions that allow surgery, clinical follow up and obtaining biological samples in these models. |
 |
The JUMISC Animal Housing Unit 22 provides services to companies and research groups in many preclinical trials. This unit advises designs and conducts preclinical validation on demand. It has qualified professionals to design, monitor and validate any type of biomedical study. In addition this unit has been certified by the Spanish Agency of Medicines and Health Products (AEMPS) for studies in “Dosing of test substance and non-clinical specimen drawing” and “Biocompatibility studies of medical devices”. These two new certifications are attached to existing “in vivo toxicity”, “Tolerance” and “Pharmacodynamics”. All these certifications allow NANBIOSIS members to perform studies to verify the effectiveness, safety and biocompatibility of nanotechnology under development. A most singular fact makes this unit is the possibility of housing, maintaining and supervising large animals for preclinical validations. This Service has extensive experience in handling and care of minipigs and what it entails use in preclinical studies. Its perimeter area is 3,200 m2, and the net floor area is 2,865 m2. It has large rooms to house the experimental animals according to their species characteristics (age, sex, weight, breed) and to the research projects they are assigned to. Among the equipment and facilities include: rooms for the maintenance of large animals, rooms for maintaining small ruminants, barrier zone for maintenance of rodents under SPF conditions, integrated operating room within the barrier area, multi-use rooms for rodents and lagomorphs maintenance, room for receipt and handling of animals, quarantine rooms pre-experimental process, room to perform small procedures and cures and preparation rooms for large animals prior to the surgical area. This unit has facilities with a perimeter area of 3,200 m2 and it has large rooms to house the experimental animals according to their species characteristics. |
 |
The range of medical imaging capabilities available within our Unit 24 allows for large animal diagnostic imaging, with the possibility to integrate all the information on body organs,systems, specific diseases or animal models, etc., that can be obtained with different imaging systems,such as clinical grade Magnetic Resonance Imaging, ultrasonography CT and fluoroscopy. This Unit is highly focused on cardiovascular diseases imaging and therapy, offering a comprehensive cardiac minimally invasive service ranging from disease modeling to therapy evaluation. Regulatory requirements prior to introducing a new medical diagnostic or therapeutic technology in clinical practice determine a mandatory preclinical validation in animal models; an scenario for which this Unit is especially suited. |