09 Jul

NANBIOSIS Unit 25 showcased preclinical MRI advances at ISMRM 2025[...]
25 Jun
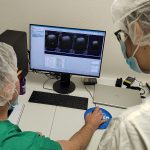
First study using NANBIOSIS Unit 26 PET/MRI‑3T shows MSC-EVs reduc[...]

Integrated solutions to advanced challenges faced by biomedical researchers in nanomedicine, tissue engineering and regenerative medicine, diagnostic and medical devices, including the design and production of biomaterials and nanomaterials and their nanoconjugates, and the characterization of these bio-/nanomaterials, tissues and medicals devices from a physic-chemical, functional, toxicological and biological point of view up to preclinical validation.
Complementing our list of “Cutting-Edge Biomedical Solutions” we offer a “Quallity Control and Regulatory Affair” Advisory Service

Design & Production of biomolecules: Customized design and production services of biological molecules for tissue engineering, intelligent devices, implants, and specially therapeutic nanoconjugates and biosensor reagents. Applications such as therapeutic agents, targeting, surface functionalization (tissue engineering or scaffolds and biosensors), and IVD reagents.
Validation: Purification, characterization, chemical modification and preservation of the developed biomolecules. Assays development and validation. Biomolecules functionalization and up-scaling.
Main CEBS are:
Design & production of nanomedicines: Supply nanomaterials, nanoconjugates and nanoencapsulation of active ingredients for applications in nanomedicine: therapy, drug delivery, contrast agents (MRI, fluorescence) , theragnostic and reagents.
Preclinical Validation: Preclinical characterisation of nanomedicines including physicochemical properties, in vitro and in vivo biological properties. immunology, toxicology and efficacy with appropriate animal models, at either regulatory (cGLP) or non-regulatory conditions. Biodistribution and metabolization follow up in animal models by NMR spectroscopy in tissues and biofluids.
Main CEBS are:
Design & Production of biomaterials: Design and production of scaffolds for tissue engineering using 3D printing technologies and others.
Preclinical Validation: Preclinical validation of biomaterials, implants and surface coatings including surface and mechanical characterization and in vitro, biofilm and antibacterial properties studies of implants and in vivo biological properties with appropriate animal models, at either regulatory (cGLP) or non-regulatory conditions.
Main CEBS are:
In vitro diagnostics & biomarkers & organ-on-a chip: Development and validation of prototypes, biosensing devices, organ-on-a chip devices for diagnostic. Production of bioreceptors against identified biomarkers. Discovery of biomarkers for diagnostic, follow-up and prognostic of diseases by NMR spectroscopy in biofluids and tissues.
In vivo Bioimaging diagnostics & Validation: In vivo validation of contrast agents and development of nanoconjugates as contrast agents for MRI and fluorescence.
Main CEBS are:
NANBIOSIS is managed under the ISO9001 certification for standard quality control system:
Order request Access protocolSurvey
Platform Unit 1.Biomolecules
|
Platform Unit 2.Biomaterials
|
Platform Unit 3.Preclinical
|
||
|
||||
Units
Scientists linked to ICTS
Projects since 2008
Services since 2008
Publications since 2008
M€ investment