Accelerate your nanomedicine’s journey to market with NANBIOSIS: tailored in vitro characterization services ensuring preclinical validation success, saving crucial time in the development process. Scientific Leaders of the project: Ibane Abasolo, José Luis Pedraz, & Ramón Mangues. One of the causes that increases the time required to reach the market in nanotherapeutics is the preclinical validation. For this to be successful, the nanomaterials needs to be tested under controlled conditions, such as in vitro, prior to start their in vivo testing. This is a crucial step since the standard protocols used for more traditional drugs are not applicable in the case of nanomedicines. NANBIOSIS offers a complete study plan tailored to meet the demands of each nanomedicine product, from the properties of the materials all the way to their preclinical validation. This plan is developed in collaboration with the user and starting from a set of basic analytical tests to more sophisticated experiments.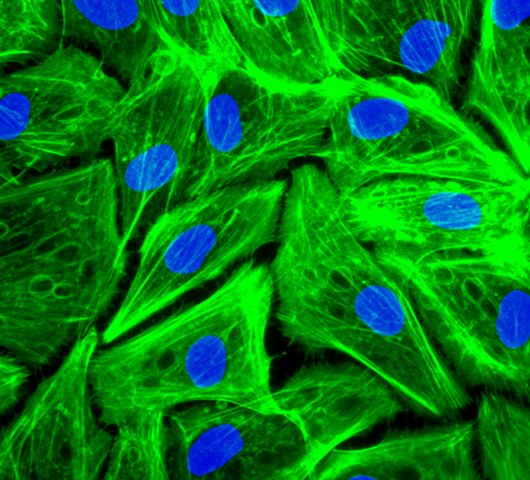
Coordinator of the project: Ibane Abasolo & Esther LópezIndustrial problem/gap covered
We offer a complete in vitro Characterization. e.g., immunology, cytotoxicity, hematology, oxidative stress, etc., and more adapted to the different products by using the most sophisticated equipment and taking advantage of the expertise of scientist internationally recognized in the matter.
Some examples are described in the following publications:
Services involved:
| Sterility | Gel-Clot LAL Assay | U20 |
| Detection of Microbial Contamination | U20 | |
| Detection of Bacterial Contamination | U20 | |
| Detection of Mycoplasma Contamination | U20 | |
| Immunology | Analysis of Hemolytic Properties of Nanoparticles | U20 |
| Analysis of Platelet Aggregation by Cell Counting | U20 | |
| Analysis of Platelet Aggregation by Light Transmission Aggregometry | U20 | |
| Analysis of Nanoparticle Interaction with Plasma Proteins by 2D PAGE | U20 | |
| Leukocyte Pro life ration Assay | U14 | |
| Detection of Nitric Oxide Production by RAW 264. 7 MacrophageCell Line | U18 | |
| ChemotaxisAssay | U18 | |
| PhagocytosisAssay | U18 | |
| Analysis of Cytokines, Chemokines and Interferons (by Elisa or Multiplex) IL-8, IL-1b, TNF-α, IFN- ϒ and many others | U14 | |
| Determination of cytokine concentration by flow cytometry | U14 | |
| Measurement of Nanoparticle Effects on Cytotoxic Activity of NK Cells by Label-Free RT-CES System | U14 | |
| In vitro Induction of Leukocyte Procoagulant Activity by Nanoparticles | U20 | |
| Oxidative Stress | Hep G2 Hepatocyte Glutathione Assay | U20 |
| Hep G2 Hepatocyte Lipid Peroxidation Assay | U20 | |
| HepatocytePrimary ROS Assay | U20 | |
| Cytotoxicity (necrosis) | Cytotoxicity (necrosis) Assay (MTT and LDH Release): LLC-PK1 Kidney, Hep G2 Hepatocarcinoma, etc | U10, U18 & U20 |
| Cytotoxicity (apoptosis) | Cytotoxicity (apoptosis) (Caspase 3 Activation) LLC-PK1 Kidney; Hep G2 Hepatocarcinoma ; etc | U10 & U18 |
| Cytotoxicity (apoptosis) (Caspase 3/7 Activation) Hep G2 Hepatocarcinoma ; etc | U10 & U18 | |
| Autophagy | Autophagic Dysfunction Assay: Qualitative Analysis of MAP LC3I to LC3-II Conversion by Western Blot | U18 |
| Autophagic Dysfunction in LLC-PK1 Cells | U18 | |
| Immunology | Lymphocyte proliferation | U14 |
| Detection of Antibodies (anti PEG and others) | U2 | |
| Cell uptake | By cytometry | U14, U18 & U20 |
| By Confocal Microscopy | U2 | |
| Of metallic NPs/Fe-staining | U20 | |
| Optical Analysis: HR Dark-Field optical microscope with spectral analysis(Nano-scale Optical Microscope) | U12 |
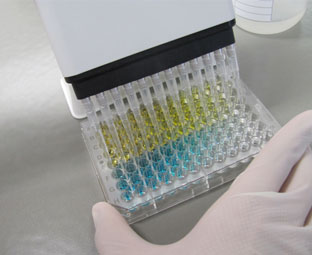
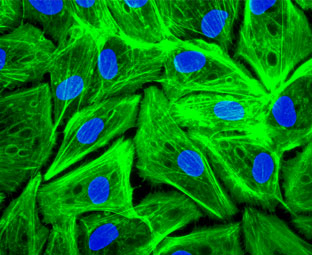
Effect of NP son cell viability and distribution in the cell cycle. Elisa plate. Phalloidin and DAPI staining of human pigmented epithelial cells (ARPE cells).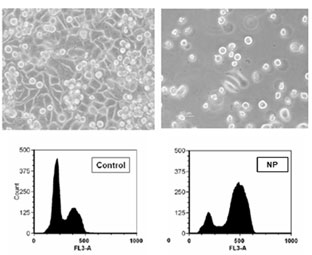
 |
CAbS (U2) is managed by the scientific team of the Nb4D group (Nb4D) with high expertise in hapten synthesis, conjugation and antibody production.We have an extensive track record of successful projects in developing polyclonal and monoclonal antibodies against small molecules and peptidesfor the development of diagnostic tools. These include, for example, the evaluation of carcinogenic processes, cardiovascular diseases, and infectious diseases. |
 |
Located at the Faculty of Pharmacy, University of the Basque Country (UPV/EHU), Campus of Alava in Vitoria. Our Unit 10 is led by Prof. José Luis Pedraz and consists of large laboratories for cell culture, chromatography equipment, sample preparation and characterization, and one specific for scale preparation of pharmaceutical formulations. Recently the group has incorporated 3D bioprinters of the last generation with different technologies based on extrusion, inkjet, among others. It has also incorporated self-assembly equipment of nanoparticles based on microfluidic technologies.
This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation. The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products. The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process. One of the singularities of this Units is that is GLP certified by the Spanish Medicament Agency |
 |
Our Unit 14 consists of a laboratory with an area of 81,02 m2 for cell biology and molecular biology techniques. The laboratory is also equipped with two cell culture rooms for the isolation, expansion and characterization of different cell types. |
 |
Unit 18 (Nanotoxicology) is located in the Hospital de la Santa Creu i Sant Pau, in Barcelona, and is coordinated by Dr. Ramón Mangues, PI of the Oncogenesis and Antitumor Drug Group. The main objective of the Nanotoxicology Unit is to assess the toxicity of new drugs, nanoparticles or nanotechnology-based biomaterials in in vitro and in vivo systems, with the goal of optimizing lead compounds and identifying those with the highest probability of success in the preclinical programme due to their greater safety and tolerability or reduced toxicity. The Unit has rooms equipped for cell culture, for cryopreservation of samples and cell lines, and for sample preparation and analysis and animal facilities for in vivo experimentation.
Equipment: The Unit has access to flow cytometers, sorters, confocal microscope and other equipment of the Platforms available at the Hospital Research Institute as well as housing facilities for small rodents (rat and mouse). |
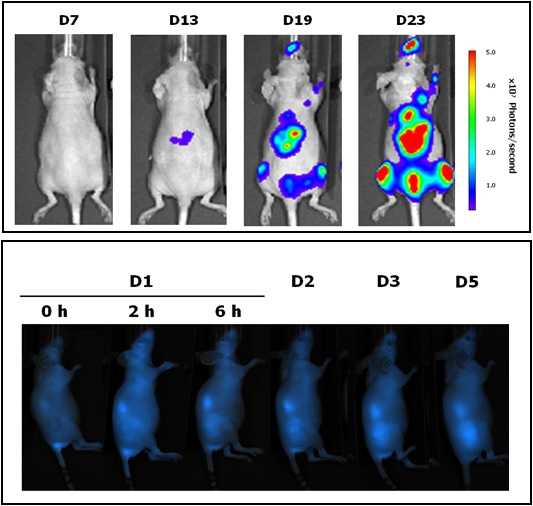 |
Located at the Valld’Hebron Research Institute (VHIR) in Barcelona, our In vivo Experimental Platform (U20) has three different sections, a Molecular Imaging section for in vivo, ex vivo and in vitro imaging studies (fluorescence, bioluminescence and X-rays), a preclinical animal model section and a preclinical histology section. All three sections are included within the Functional Validation & Preclinical Research (FVPR) of CIBBIM-Nanomedicine.
This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation. The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products. The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process. One of the singularities of this Units is that is GLP certified by the Spanish Medicament Agency |