Develop and validate customized 3D-bioprinted scaffolds with in vivo models, bridging advanced biomaterials research and real-world regenerative medicine applications.
Scientific Leader / leaders of the project: José Luis Pedraz / Francisco M. Sánchez Margallo
Coordinator of the project: Idoia Gallego / Ángela Losada / Francisco M. Sánchez Margallo
Biomaterials for regenerative purposes are one of the clearest examples of targeted medicine. The rapid advance of tissue engineering and regeneration field, has focused the attention on 3D bioprinting as the main technique to successfully mimic the target tissue, by using specific cell types, biomaterials and polymers, while locally delivering therapeutic agents or bioactive molecules of interest. In this sense, one of the main characteristics of this additive manufacturing technique is its ability to bioprint the desired layers, with specific cell orientation, and desired morphology of the bioprinted 3D scaffold in order to resemble, as much as possible, the mechanical and biological properties of the target tissue for its regeneration.
This cutting-edge approach opens up the possibility of developing by 3D bioprinting the desired tissue scaffolding with full characterization and assessing the in vivo functionality of the graft in both small and large animal models.
Examples of scientific publications implementing this Cutting-Edge Biomedical Solution:
Examples of projects implementing this Cutting-Edge Biomedical Solution:
Services involved:
These studies are carried out by the U10 (Drug Formulation Unit) at the University of the Basque Country (UPV/EHU) and the U14, U21, and U22 (Cell Therapy Unit, Experimental Operating Rooms Unit and Animal Housing Unit respectively) at the Jesús Usón Minimally Invasive Surgery Center (JUMISC). These four units are currently collaborating on projects of this type for tendon and bone regeneration purposes. The steps and services involved in this cutting-edge solution are the followings:
 |
Located at the Faculty of Pharmacy, University of the Basque Country (UPV/EHU), Campus of Alava in Vitoria. The Unit 10 is led by Prof. José Luis Pedraz and consists of large laboratories for cell culture, chromatography equipment, sample preparation and characterization, and one specific for scale preparation of pharmaceutical formulations. Recently the group has incorporated 3D bioprinters of the last generation with different technologies based on extrusion, inkjet, among others. It has also incorporated self-assembly equipment of nanoparticles based on microfluidic technologies. This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation. The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products. The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process. |
 |
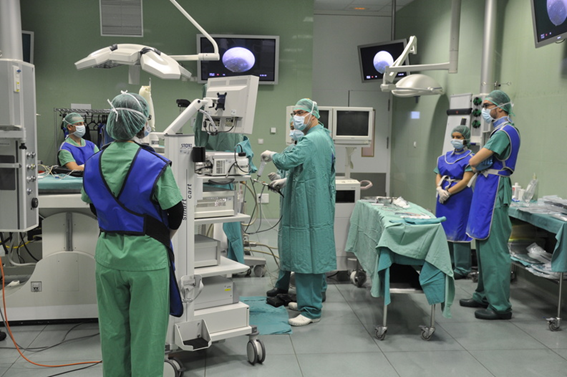
Our Unit 21 is equiped with experimental operating rooms with high technology and high field instrumentation to promote methodological and translational research class in Biomedicine, both minimally invasive surgery and new procedures and in preclinical development for technical evaluation, medical devices, biomaterials, etc. Surgical facilities allow the development and use of small and large animal models for research and experimental surgery in several longitudinal areas: cardiovascular diseases, liver and digestive diseases, respiratory and systemic diseases, endocrinology and urology, gynecological and reproductive diseases, pediatric diseases, orthopedics and traumatology, ophthalmology and experimental surgery in all organ systems have the infrastructure for transplant development, implants, prosthesis, biomaterials, etc. under high quality experimental conditions that allow surgery, clinical follow up and obtaining biological samples in these models. |
 |
The JUMISC Animal Housing Unit (NANBIOSIS Unit 22) provides services to companies and research groups in many preclinical trials. This unit advises designs and conducts preclinical validation on demand. It has qualified professionals to design, monitor and validate any type of biomedical study. In addition this unit has been certified by the Spanish Agency of Medicines and Health Products (AEMPS) for studies in “Dosing of test substance and non-clinical specimen drawing” and “Biocompatibility studies of medical devices”. These two new certifications are attached to existing “in vivo toxicity”, “Tolerance” and “Pharmacodynamics”. All these certifications allow NANBIOSIS members to perform studies to verify the effectiveness, safety and biocompatibility of nanotechnology under development. A most singular fact makes this unit is the possibility of housing, maintaining and supervising large animals for preclinical validations. This Service has extensive experience in handling and care of minipigs and what it entails use in preclinical studies. |
 |
Unit 14 consists of a laboratory with an area of 81,02 m2 for cell biology and molecular biology techniques. The laboratory is also equipped with two cell culture rooms for the isolation, expansion and characterization of different cell types. |