
 |
  |
The Nanoimaging Unit is located in Malaga at the Andalusian Centre for Nanomedicine and Biotechnology (BIONAND), and it has been conceived and optimized to provide very diverse and integrated technological support for nanotechnology-based biomedical research, offering advanced services to internal and external users. This facility is coordinated by Dr Mª Luisa García Martín, who has wide expertise in the field of Magnetic Resonance Imaging and Spectroscopy applied to biomedical research.
The Unit brings together a wide range of cutting-edge technologies, and it is divided into four departments, each with a dedicated specialist:
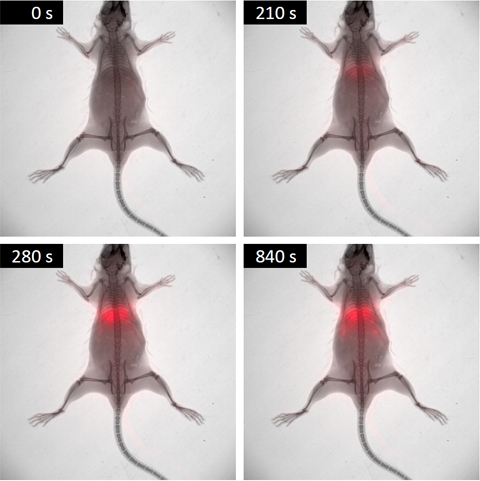
– Small Animal Multimodal Imaging: Small animal (pre-clinical) imaging is a vital tool in biomedical research that allows biological processes and potential medicines to be studied in animal models using non-invasive methods. This department offers some of the most relevant pre-clinical imaging modalities, namely, MRI, in vivo Bioluminescence and Fluorescence Imaging, and micro-CT. These techniques are essential for preclinical evaluation and characterization of new diagnostic and therapeutic agents. The combined use of these technologies and new highly specific imaging probes are the basis of molecular imaging.
– Magnetic Resonance (NMR): it is equipped with two High-resolution NMR Spectrometers: a 400MHz NMR spectrometer for routine experiments and a higher field, 600 MHz, spectrometer dedicated to high-resolution magic angle spinning (HR-MAS) NMR and in vitro studies that are very demanding in terms of sensitivity. It also has a TD-NMR analyzer for relaxivity measurements in the clinical field (1.44 T).
– Electron Microscopy: It offers access to transmission electron microscopy (TEM), Cryo-TEM and Electron Tomography, high-resolution scanning electron microscopy (SEM) and environmental SEM. A special emphasis has been made on high-end sample preparation techniques through cryo-immobilization. All the equipment has been configured to provide its best on biological and nanomedicine applications. TEM, SEM and sample preparation facilities and services are offered to provide structural characterizations at the micro and nano scales. Biological and medical applications are the main objective of this equipment but are not limited to them. Any other scientific and engineering field can take advantage of them due to their versatility for public and private research, quality control, failure analysis, etc.
– Advanced Fluorescence: It hosts an excellent range of facilities capable of supporting high-quality biomedical research. These applications can range from the initial characterization of the fluorescent properties of novel nano-compounds to the evaluation of their medical applications using tissue culture and animal models. In addition, the range of possible applications goes far beyond nanomedicine to include the characterization of patient tissues, preclinical studies using rodent models, small-scale drug screening and studies using model organisms such as Zebrafish or Drosophila Melanogaster. The service is operated in close collaboration with the other advanced facilities of the Nanoimaging Unit, and the other units of Bionand, to ensure that researchers can easily combine fluorescence-based techniques with other complementary approaches such as Electron microscopy or Nuclear Magnetic Resonance.
Moreover, the location of the NanoImaging Unit allows it to be linked to other complementary Units available at BIONAND, such as the Cell Culture Unit (containment levels 1 and 2), the Chemical Unit (it offers a wide range of equipment and techniques in which materials and their interaction with biological samples can be characterized), the Animal House (rat, mouse and zebrafish accommodation), and the Histology Unit (it also offers Cutting/grinding based histological sectioning for hard tissues or other materials unsuitable to be sectioned by routine methods)
In addition to the services within the NanoImaging Unit, the following specialised services are offered:
– Cutting/grinding based histological sectioning (Onsite&Remote) OUTSTANDING: This technique is recommended for obtaining histological samples from hard tissues or other materials unsuitable to be sectioned by routine methods, e.g. undecalcified bone tissue, metallic or ceramic implants, or samples that combine hard and soft components. With the EXAKT system, 10-15 μm slices can be made without disrupting the interface between areas of different hardnesses. Applications: Processing and fixation of fresh samples, Dehydration, plastic infiltration and polymerization, Cutting of blocks to obtain surfaces of interest, Block grinding, and Histological staining.
– Animal Housing (Onsite&Remote): The BIONAND Animal Facility Service is a research support service that aims to house experimental animals: zebrafish, rats and mice, and provide the facilities, materials, equipment and expertise needed to conduct pre-clinical trials based on the technologies and products developed in Centre from both research groups and companies
| Ref | Title | Funding Organism | Unit Role |
|---|---|---|---|
| T96 | BIOMEDICAL SIGNAL INTERPRETATION & COMPUTATIONAL SIMULATION | DGA | Participant |
| UZCUD2016-TEC-03 | Optimization of a submersible device for the monitoring of the photoplestimográfica signal | CUD | Participant |
| DPI2016-75458-R | MULTI-SCALE PHYSIOLOGY-DRIVEN COMPUTATIONAL TOOLS TO ASSIST IN THE ASSESSMENT AND MANAGEMENT OF CARDIAC DYSFUNCTIONS | MINECO | Participant |
| TIN2013-41998-R | THE AUTONOMOUS NERVOUS SYSTEM AS A MODULATOR OF CARDIAC FUNCTION: INTEGRAL RESEARCH THROUGH SIGNAL PROCESSING AND COMPUTATIONAL MODELING | MINECO | Participant |
Tags: biomarker nanomedicine