NANBIOSIS Strengthens Internal Collaboration at 2025 Networking Event in Cáceres
NANBIOSIS held its 2025 networking event in Cáceres to boost collaboration among Units in biomaterials, nanomedicine, and biomedical research.
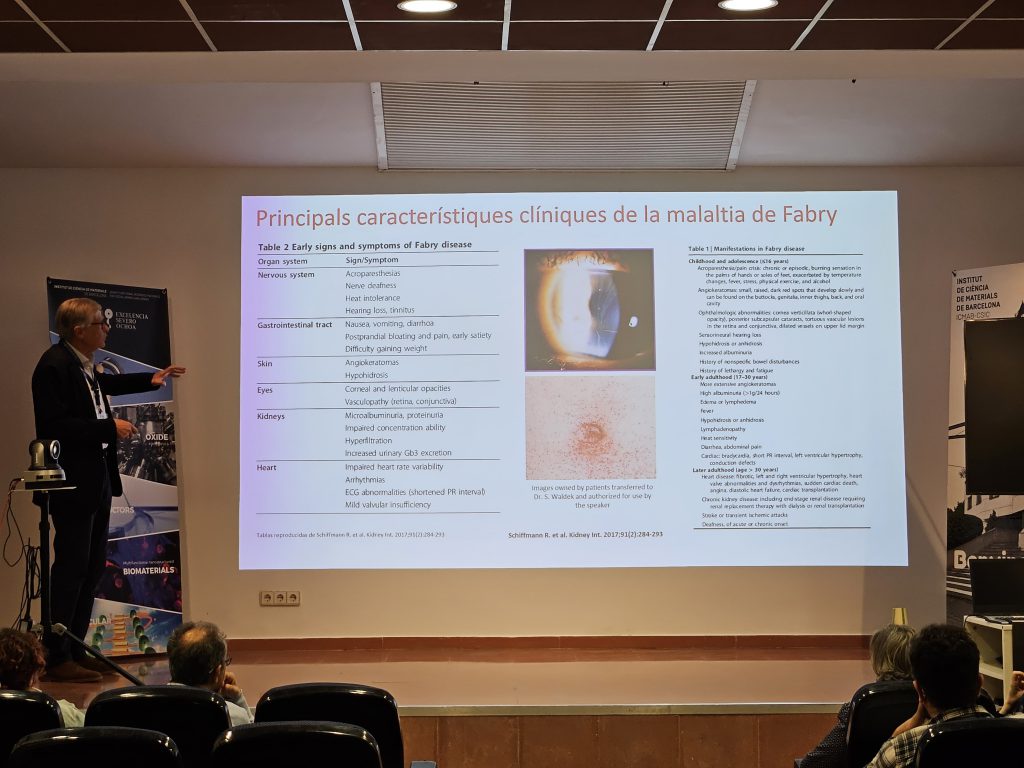
Cáceres, October 2025 — From 22 to 24 October 2025, the Singular Scientific and Technical Infrastructure (ICTS) NANBIOSIS held its annual internal networking event at the Centro de Cirugía de Mínima Invasión Jesús Usón (CCMIJU) in Cáceres, Spain. The event brought together Unit coordinators, researchers, and technical staff from across Spain to foster collaboration, share scientific progress, and explore new opportunities for joint projects in biomedical research, nanomedicine, and biotechnology.
A meeting to boost collaboration across NANBIOSIS Units
The three-day event was designed to enhance synergy among all the Units that make up NANBIOSIS, a distributed infrastructure offering cutting-edge services for biomaterials, nanomaterials, bioimaging, preclinical validation, and high-performance computing.
The meeting opened on 22 October with a welcome address by the Secretary General for Science of the Junta de Extremadura, who highlighted the strategic importance of research infrastructures like NANBIOSIS for innovation in health technologies. This was followed by an introduction to the initiative “Cutting Edge Biomedical Solutions”, reinforcing the mission of NANBIOSIS to support advanced biomedical development.
That afternoon, participants presented their work grouped under three scientific programmes:
- Programme 1: Production of Biomolecules (Units 1 to 3 and 29)
- Programme 2: Production of Biomaterials and Nanomaterials (Units 6 to 10)
- Programme 5: High-Performance Computing (Unit 27)
The day concluded with a networking session encouraging informal exchanges and inter-Unit dialogue, in the context of the outstanding facilities of CCMIJU, one of the three nodes that form NANBIOSIS.
Guided Tour and Technical Presentations at CCMIJU
On 23 October, participants took part in a guided tour of CCMIJU, visiting the centre’s advanced research facilities in preclinical studies, imaging technologies, and tissue engineering.
After a short coffee break, the morning continued with presentations from Programme 3: Preclinical Validation – Characterisation of Tissues, Biomaterials and Surfaces (Units 12 to 19, and 30). Finally, the last programme was explained that same day in the afternoon, with Programme 4: Preclinical Validation – Bioimaging (Units 20 to 26, and 28).
Similarly to the previous day, throughout the day networking sessions facilitated direct interaction between teams, helping identify new collaboration opportunities across scientific programmes.
To close the technical sessions, participants joined “The NANBIOSIS Contest”, an interactive Kahoot-style quiz promoting engagement and friendly competition. In the evening, a guided cultural visit of Cáceres allowed attendees to explore the historic city’s UNESCO World Heritage old town.
Strengthening the NANBIOSIS Network
The final day, 24 October, saw participants returning to Madrid, concluding an event marked by enthusiasm, collaboration, and a strong sense of community across the infrastructure.
This internal networking event successfully:
- Reinforced communication and coordination among NANBIOSIS Units.
- Promoted awareness of each Unit’s services, equipment, and expertise.
- Laid the groundwork for new interdisciplinary collaborations and new CEBS.
- Highlighted the role of NANBIOSIS as a national and international reference in biomedical innovation and nanotechnology.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: