
Fast-track nanotherapeutics to market with NANBIOSIS – expert characterization studies tailored for nanomedicines ensure rapid regulatory approval.
Scientific Leaders of the project: Nora Ventosa, Jesús Santamaría, José Luis Pedraz & Carlos Rodríguez.
Coordinator of the project: Jaume Veciana
Services involved: Standar services
Others
Some examples are described in the following publications:

U6. Dynamic light scattering for particle size and Z potential measurements.

U10. Dissolution_rate_determination_equipment

U12-E02. CytoViva Hyperspectral Imaging System (HSI).
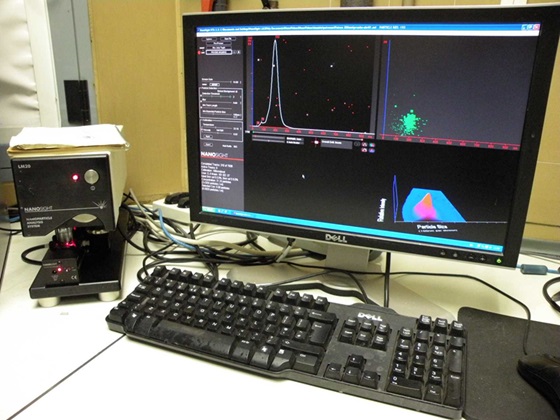 |
Our Unit 6 is located at the Instituto de Ciencia de Materiales de Barcelona (ICMAB-CSIC), in Barcelona, and under the coordination of Professor Jaume Veciana and Prof. Nora Ventosa, current directors of NANOMOL Group, which is a research group with wide expertise and recognized excellence in the synthesis, processing and study of molecular and polymeric materials with chemical, electronic, magnetic and biomedical properties. It gathers several laboratories, perfectly equipped, to perform the mission of this facility: the development, characterization, and large-scale production of molecular biomaterials of therapeutic or biomedical interest, with controlled micro-, nano- and supramolecular structure. One example of Key-Enabling-Technology (KET) available in this unit is a simple one-step methodology, DELOS-SUSP, based on the use of compressed fluids (CF), such as CO2, to prepare particulate materials with precise and reproducible structural characteristics at micro-, nano- and supramolecular levels (size, shape, internal structural gradients, supramolecular organization and crystalline purity). This example shows one of the singularities of this unit is that counts with CF–based plants at different scales, from mL to L, which allow process development by QbD and process scale-up. The NANBIOSIS Unit 6 is works under the ISO9001 certification for standard quality control system
|
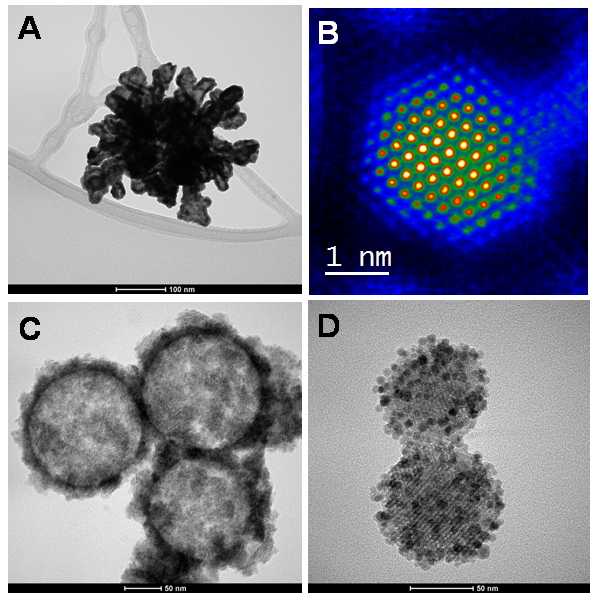 |
Unit 9, integrated in the Nanostructured Films and Particles Group at the Institute of Nanoscience of Aragón (INA) coordinated by Dr. Jesús Santamaría, has as objective the synthesis of nanoparticles with applications in biomedicine. The unit provides an automated system for the synthesis of nanoparticles using laser-induced pyrolysis of chemical precursors in gas and/or aerosol phase, which enable either individual nanoparticles or biocompatible hybrid nanostructures to be produced in large quantities. In addition, this facility is able to draw on a wide range of nanoparticles fabrication technique, as well as having the necessary specialized personnel, to undertake exhaustive characterization of the microstructure, chemical composition, particles size and distribution of sizes, as well as magnetic, optical and colloidal properties and degree of biological functionality of the synthesized material. |
 |
Located at the Faculty of Pharmacy, University of the Basque Country (UPV/EHU), Campus of Alava in Vitoria. Our Unit 10 is led by Prof. José Luis Pedraz and consists of large laboratories for cell culture, chromatography equipment, sample preparation and characterization, and one specific for scale preparation of pharmaceutical formulations. Recently the group has incorporated 3D bioprinters of the last generation with different technologies based on extrusion, inkjet, among others. It has also incorporated self-assembly equipment of nanoparticles based on microfluidic technologies. This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation. The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products. The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process. One of the singularities of this Units is that is GLP certified by the Spanish Medicament Agency |
 |
Our Unit 12 is located at the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC), in Barcelona and it is coordinated by the Colloidal and Interfacial Chemistry Group, led by Dr. Carlos Rodríguez. The unit is focused on the characterization of nanostructures in liquids and liquid/liquid interfaces, such as micelles, liposomes, micro- and nano-emulsions, and nanoparticles. The unit features state-of-the-art instrumentation for the determination of various parameters such as size distribution and zeta potential (surface charge), critical micelle concentration (CMC), solubilization capacity, osmolarity, surface and interfacial tension, wettability, refractive index, turbidity, colloidal stability, density, viscosity and other rheological parameters (shear and elastic moduli, critical yield stress, etc.) The Unit 12 has implemented and maintains the ISO9001 certification forstandard quality control system.
|