New Consequences in Familial Hypercholesterolemia due to Abnormal LDL
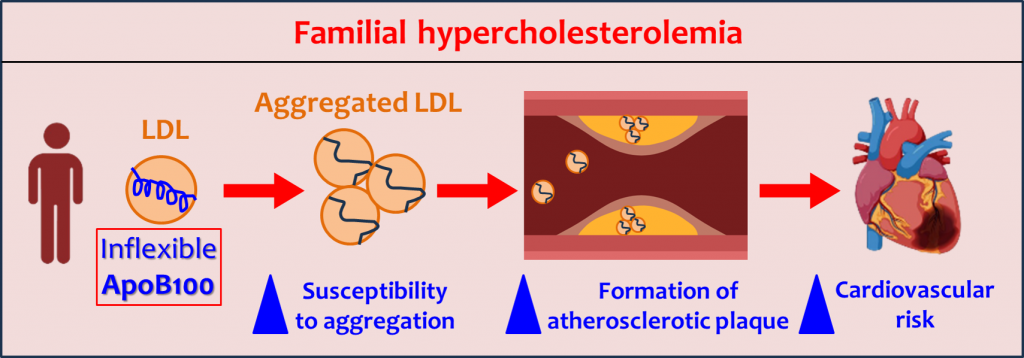
Misfolded ApoB100 in LDL promotes plaque buildup in familial hypercholesterolemia, raising cardiovascular risk. Structural preservation may aid prevention.
Barcelona, febrero 2025. As recently published in the ICMAB webpage, a new study links ApoB100 protein structure (a key protein found in low-density lipoprotein, LDL, often called “bad cholesterol”) to increased cardiovascular risk in familial hypercholesterolemia. The study, led by IIBB-CSIC and CIBER, with the contribution of ICMAB’s SOFT Service (with NANBIOSIS Unit 6 at its core), shows that the protein structure directly contributes to an increased tendency to aggregate and form atherosclerotic plaques in patients with familial hypercholesterolemia.
In these patients, LDL particles are smaller due to their high content of esterified cholesterol, and ApoB100 has a less flexible structure due to its high percentage of rigid alpha-helices. Developing strategies to preserve the structure of ApoB100 could be a new way to reduce the risk of cardiovascular disease in these patients.
ApoB100 protein structure drives LDL accumulation and increases cardiovascular risk, study finds
This new multicenter study reveals why the structure of the ApoB100 protein, present in LDL along with the so-called “bad cholesterol,” plays a crucial role in the tendency of LDL to accumulate in the arterial walls of patients with familial hypercholesterolemia, thus promoting the formation of atherosclerotic plaques.
The study is led by Vicenta Llorente Cortes, a researcher at the Biomedical Research Institute of Barcelona (IIBB-CSIC) and CIBER-CV, and Valerie Samouillan, a researcher at the University of Toulouse Paul Sabatier.
Familial hypercholesterolemia is a fairly common genetic disorder, affecting about 1 in every 200 or 300 people. Those affected have high levels of low-density lipoprotein (LDL) cholesterol from birth and consequently have a higher risk of cardiovascular diseases and greater rates of premature death due to these conditions.

But, why do LDL particles aggregate more in these individuals? Are there biochemical and physical differences that explain this? This is what the study, published a few weeks ago in the Journal of Lipid Research, aimed to clarify. The study involved 10 research centers in Spain and France. These include, in addition to IIBB-CSIC and CIBER, the Institute of Materials Science of Barcelona (ICMAB-CSIC), the Sant Pau Research Institute (IR Sant Pau), the Autonomous University of Barcelona (UAB), the CIRIMAT Institute (Toulouse, France), and the Miquel Servet Hospital in Zaragoza.
ApoB100 Structure: Less flexible in small, dense LDL particles in patients with familial hypercholesterolemia
LDL particles in patients with familial hypercholesterolemia show a greater tendency to aggregate and form plaques. This is due, explains Vicenta Llorente Cortes, “to the fact that the ApoB100 protein in LDL has a particular structural conformation, with a high percentage of rigid alpha-helices [secondary structures], compared to LDL from healthy patients.”
Using samples from 35 patients with familial hypercholesterolemia and 29 healthy individuals as a control group, the researchers demonstrated that in patients with familial hypercholesterolemia, the protein present in LDL is smaller due to its high content of esterified cholesterol and has less structural flexibility compared to LDL from healthy individuals. As a result, these LDL particles have a lower ability to recover their structure at the arterial intima, promoting their accumulation on the inner walls of the arteries.
Various Techniques to Study LDL Particles
The study measured, among other things, the ease with which LDL particles aggregate using dynamic light scattering techniques (measured at the SOFT Service at ICMAB-CSIC, Unit 6 of NANBIOSIS), as well as the size, composition, and structure of LDL particles through electron microscopy.
As Llorente explains, one of the most impactful findings was the discovery of the difference in the percentage of flexible secondary structures in ApoB100 from patients with familial hypercholesterolemia, which would not have been possible without the collaboration of the biophysics group led by Valerie Samouillan (University of Toulouse). This group applied the FTIR infrared spectroscopy technique to determine the protein’s structure and, in particular, quantify the stable alpha-helices and flexible alpha-helices in LDL from control and patient groups.
The results suggest that developing strategies to structurally preserve ApoB100, and in particular the percentage of flexible alpha-helices in LDL, could be a new way to reduce the risk of cardiovascular disease in patients.
This finding offers a new perspective on how alterations in ApoB100 structure can directly influence the risk of developing cardiovascular diseases. Furthermore, it opens possibilities for designing specific therapies aimed at modulating the content of flexible alpha-helices in LDL, contributing to the prevention of atherosclerosis.
“With these new peptide tools, we aim to preserve the structural flexibility of the ApoB100 protein in LDL from patients with familial hypercholesterolemia.”
Vicenta Llorente
“In our research group,” adds Vicenta Llorente, “we are comparing whether PCSK9 inhibitors [a type of drug] can help preserve the percentage of flexible alpha-helices and whether these effects are comparable to those achieved through innovative peptide tools developed in our group specifically for this purpose. With these new peptide tools, we aim to preserve the structural flexibility of the ApoB100 protein in LDL from patients with familial hypercholesterolemia.”
ICMAB and NANBIOSIS contribution
Amable Bernabé, technician at the SOFT Service of ICMAB-CSIC, and part of NANBIOSIS (Unit 6), played a key role in this study, contributing to the analysis of LDL particle size using the dynamic light scattering (DLS) (Zetasizer) technique. He also participated in the development of the analysis method, interpreting the results, discussing them, and proposing complementary techniques to make the study more robust.
The SOFT Service offers state-of-the-art equipment and technical support for the preparation and characterization of micro- and nanostructured soft molecular materials. This includes molecular surfaces, micro- and nanoparticulate materials, plastic films, dispersed systems, and self-assembled monolayers (SAMs). These materials have applications across various fields, including biomedicine, electronics, energy storage, and other chemical and materials sciences.
Reference article:
Maria Teresa La Chica Lhoëst, Andrea Martínez, Eduardo Garcia, Jany Dandurand, Anna Polishchuk, Aleyda Benitez-Amaro, Ana Cenarro, Fernando Civeira, Amable Bernabé, David Vilades, Joan Carles Escolà-Gil, Valerie Samouillan, Vicenta Llorente-Cortes.
ApoB100 remodeling and stiffened cholesteryl ester core raise LDL aggregation in familial hypercholesterolemia patients.
Journal of Lipid Research. 2025 Jan;66(1):100703. DOI: 10.1016/j.jlr.2024.100703
Watch the following video for more information:
More information about the publication can be found here. This article is a reproduction with a slight edition that does not alter the overall message.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: