U14-S05. Determination of cytokine concentration by flow cytometry (On-site&Remote) OUTSTANDING
Determination of cytokine concentration by flow cytometry
Determination of cytokine concentration by flow cytometry
Cytokine quantification assays
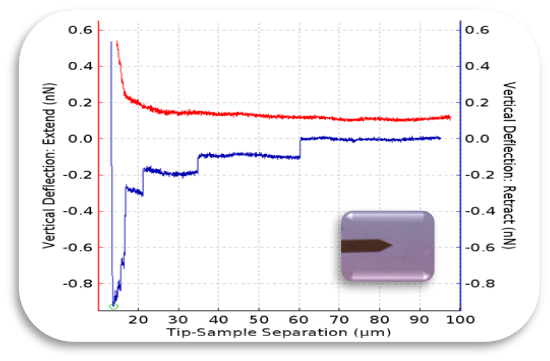
Atomic Force Microscopy can be used to measure the forces between the probe and the sample as a function of their mutual separation. This can be applied to perform force spectroscopy.
Customer benefits
Low forces on cells (nano-scale).
Target customer
Public and private research groups focused on mechanobiology
Additional information

Encapsulation of therapeutic actives of interest in formulations for pulmonary administration with possibility of lyophilisation to obtain a powder. The service has two cutting-edge equipments for the characterization of pulmonary formulations:
SprayTec laser diffraction system: allows the measurement of spray particle and spray droplet size distributions in real-time for more efficient product development of sprays and aerosols, with robust and reproducible droplet size data.
Next Generation Impactor: has been designed specifically for the pharmaceutical industry for testing metered-dose inhalers, drypowder inhalers, nebulizers and nasal sprays. It consists of a high performance cascade impactor for classifying aerosol particles into micrometer size fractions, providing relevant information about their distribution in the respiratory tract.
Customer benefits
The formulations can be characterized, following SOPs, in terms of particle size, polydisperstity index and zeta potential. Importantly, this service can also offer real-time droplet size distribution and aerosol particles classification analysis by means of SprayTec and Next Generation Impactor (NGI) technology.
Target customer
References
Moreno-Sastre M, Pastor M, Esquisabel A, Sans E, Viñas M, Fleischer A, Palomino E, Bachiller D, Pedraz JL. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2016 Feb 10;498(1-2):263-73. doi: 10.1016/j.ijpharm.2015.12.028.
Additional information
