Scientific presentation and use of the piezoelectric micromanipulator and the time-lapse incubator within the framework of the Genera Project
Cáceres, 31st January 2023
On the occasion of the training course in Assisted Reproduction that is held today at the CCMIJU facilities, within the Master’s Degree in Advanced Biotechnology (MUBA), organized by the University of Extremadura in collaboration with the CCMIJU, two systems of recent acquisition are handled within the framework of the project “Embryonic Genetics in Assisted Reproduction” (GENERA), co-funded by the European Regional Development Fund (FEDER) within the Spain’s Pluriregional Operational Program for Singular Scientific and Technical Infrastructures (ICTS) 2014-2020 and by the Consejería de Economía, Ciencia y Agenda Digital of the Junta de Extremadura.
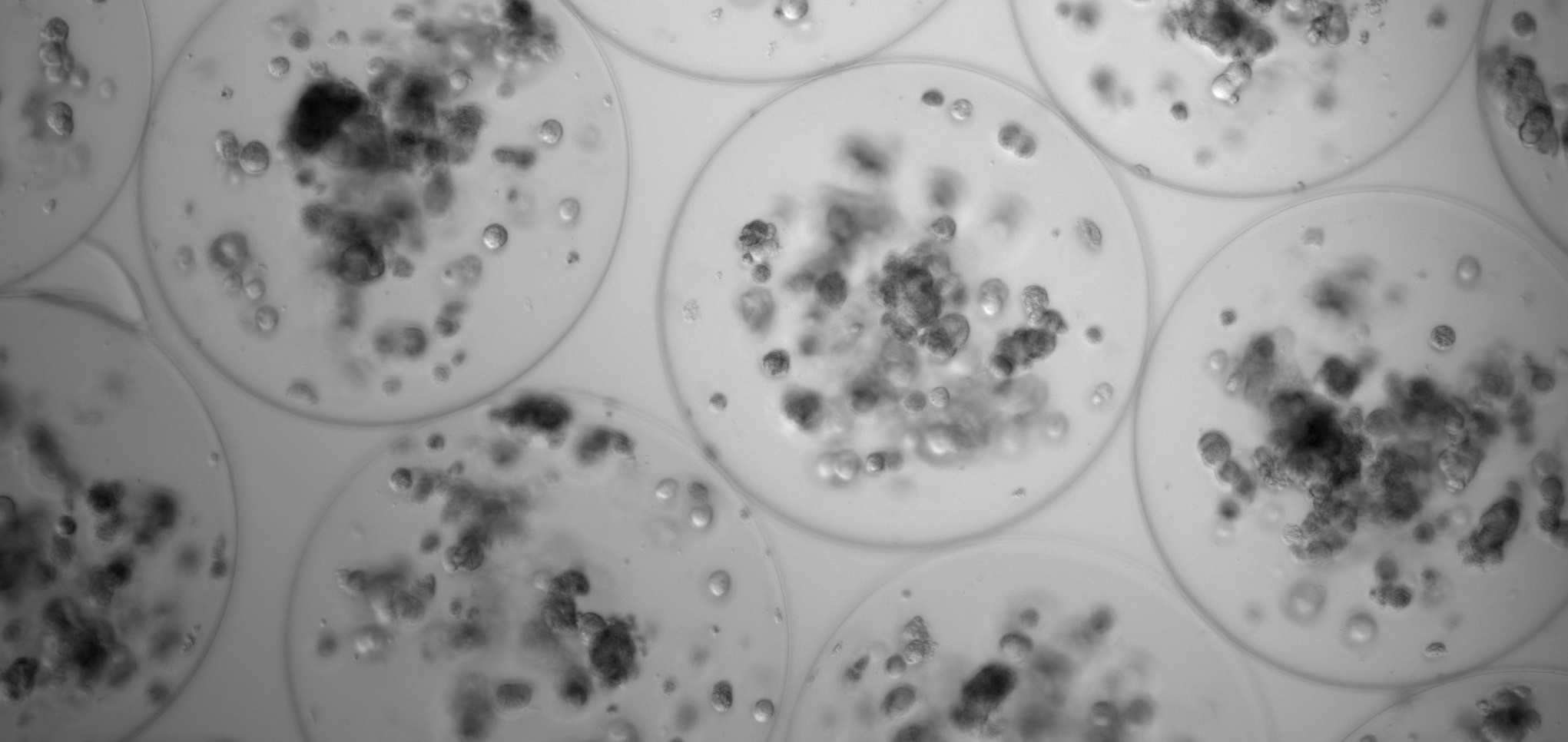
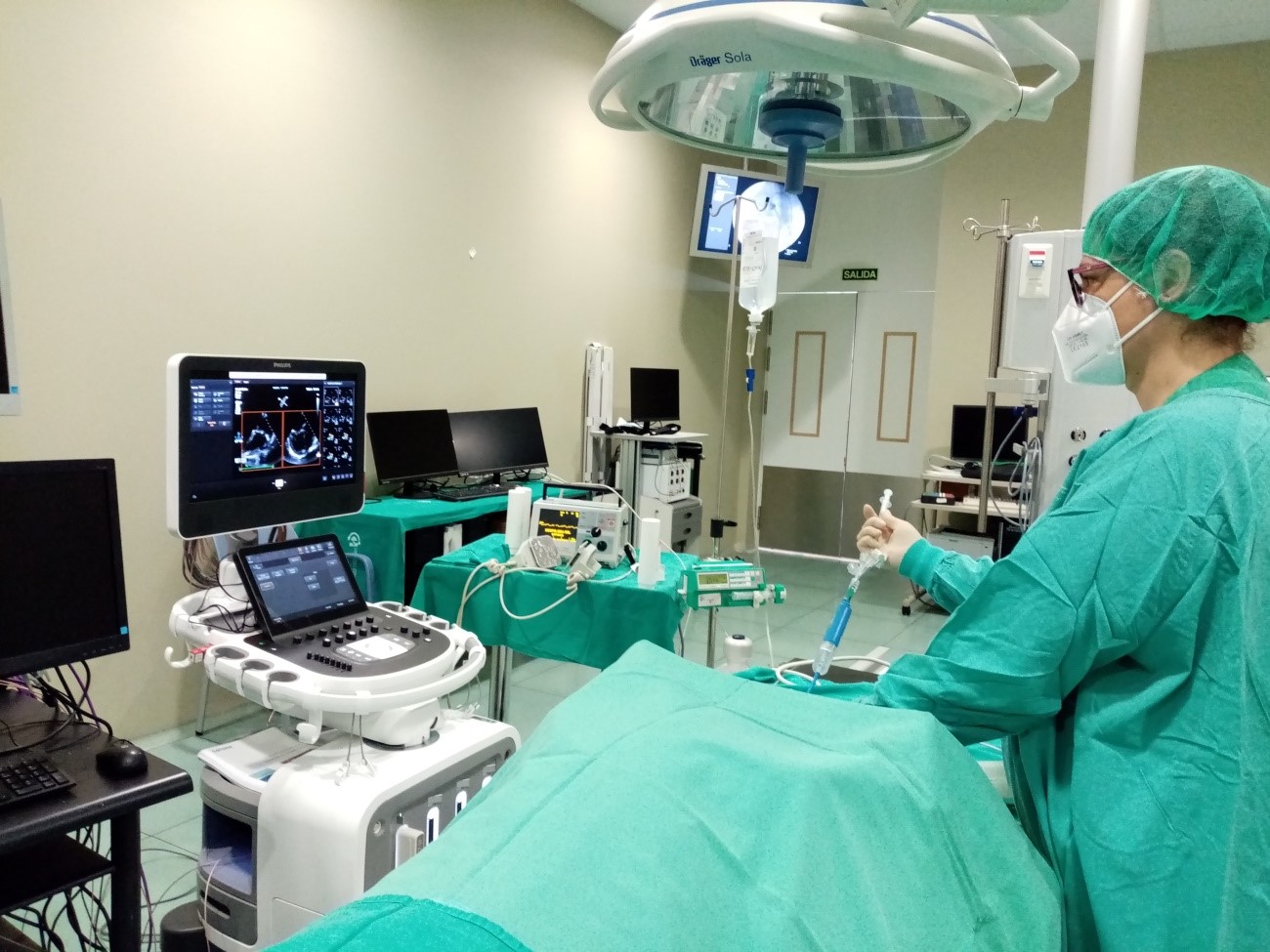
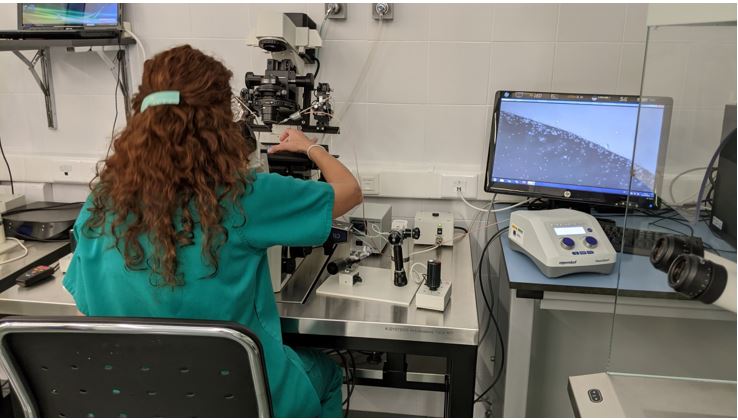
The above mentioned systems are a Piezoelectric Micromanipulator that allows the introduction of needles or capillaries into the cells for subsequent microinjection, and a Time-lapse Incubator to regularly observe embryos, perform precise evaluations, and minimize embryonic culture stress. Both are installed in the assisted reproduction laboratory of the CCMIJU and are being used by 10 master’s students to carry out their practices.
The project has an eligible budget of €98,000, of which the ERDF co-financing rate (80%) amounts to 78,400 and the national contribution to €21,600. It is expected to be completed in June 2023.