NANBIOSIS in the Posters presentation in CIBER-BBN ANNUAL CONFERENCE 2018
Last 12 and 13 of November, CIBER-BBN has celebrated its 12th Annual Conference in Hotel Felipe IV in Valladolid. In poster session participated NANBIOSIS itself and some of its units.
- NANBIOSIS, Infrastructure for the Production & Characterization of Biomaterials, Nanomaterials and medical devices up to preclinical validation. Nanbiosis management team.
- Divalent cation effects on assembly of histidine-rich protein nanoparticles. López-Laguna, U. Unzueta, O. Conchillo-Solé, A. Sánchez-Chardi, M. Pesarrodona, O. Cano-Garrido, E. Voltà, L. Sánchez-García, N. Serna, P. Saccardo, R. Mangues, A. Villaverde, E. Vázquez (NBT-UAB) (U1 -U18)
- Antimicrobial peptides (AMPs) anchored on the surface of contact lenses to prevent corneal infections. Emiliano Salvagni, Clara García, Àngels Manresa, Carlos Rodríguez, María José García-Celma, Claudia Müller-Sánchez, Manuel Reina, Jordi Esquena (QCI-CSIC) (U12)
- Preliminary studies on ultrasound characterization of perfluorocarbon-loaded polymeric nanocapsules. Gabriela Calderó, Marie Pierre Krafft, Da Shi, María José García-Celma, Conxita Solans, Carlos Rodríguez-Abreu (QCI-CSIC) (U12)
- Multiplexed analytical platforms based on the use of antibodies for monitoring pollutants in marine environment samples. -Pablo Salvador, Ana Sanchís, Klaudia Kooper, Andrea Miti, M.-Pilar Marco (Nb4D-IQAC-CSIC) (U2)
- In vitrodiagnostics of neurological disorders through the immunochemical detection of kynurenine. Montserrat Rodríguez Núñez, Ana Sanchís, Lluïsa Vilaplana, Roger Galve, M.-Pilar Marco (Nb4D-IQAC-CSIC) (U2)
- Custom Antibody Service: From the molecule to the bioassay. Núria Pascual, Ana González-Gomzález, M.-Pilar Marco (Nb4D-IQAC-CSIC) (U2)
- Hyaluronic acid enhances insulin release of microencapsulated pancreatic progenitors differentiated from mesenchymal stem cells. Alberto Cañibano-Hernández, Laura Sáenz del Burgo, Albert Espona-Noguera, Gorka Orive, Rosa María Hernández, Jesús Ciriza, Jose Luis Pedraz (NANOBIOCEL) (U10 )
- Type 1 Diabetes Mellitus reversal via implantation of magnetically purified microencapsulated pseudoislets. Albert Espona-Noguera, J. Etxebarria-Elezgarai, L. Saenz del Burgo, A. Cañibano-Hernández, H. Gurruchaga, Gorka Orive, Rosa M. Hernández, F. Benito-Lopez, J. Ciriza, L. Basabe-Desmonts and J.L. Pedraz (NANOBIOCEL) (U10 )
- Unraveling the immune system participation in therapy response in GL261 glioblastoma: correlation with MRSI-based molecular imaging techniques. Calero, N. Arias-Ramos, R. Rabanal, M. Pumarola, C. Arús, A.P. Candiota (GABRMN-UAB) (U25)
- Dual T1/T2 nanoscale coordination polymers as novel contrast agents for MRI: a preclinical study for brain tumor. Suárez-García, N. Arias-Ramos, C. Frias, A.P. Candiota, C. Arús, J. Lorenzo, D. Ruiz-Molina, F. Novio (GABRMN-UAB) (U25)
- Impact of high glucose levels and ketoacidosis associated to diabetic complications on bacterial growth and attachment to Ti6Al4V. Miguel A. Pacha-Olivenza, María Fernández-Grajera, Daniel Romero-Guzmán, M. Luisa González-Martín (AM-UEX) (U16)
- Preparation, characterization and bacterial performance on PLDA and PLDA 10% Mg films. Verónica Luque-Agudo, Daniel Romero-Guzmán, Coronada Fernández-Calderón, Miguel A. Pacha-Olivenza, María Fernández-Grajera, Margarita Hierro-Oliva, M. Luisa Navarro-Pérez, Ciro Pérez-Giraldo, M. Luisa González-Martín, Amparo M. Gallardo-Moreno (AM-UEX) (U16)
- A wavelet-based approach for automatic diagnosis of strict left bundle branch block. Alba Martín-Yebra, Juan Pablo Martínez (BSICoS) (U27)
- ECG-derived respiration in atrial fibrillation. Spyridon Kontaxis, Jesús Lázaro, Valentina D. A. Corino, Frida Sandberg, Raquel Bailón, Pablo Laguna, and Leif Sörnmo (BSICoS) (U27)
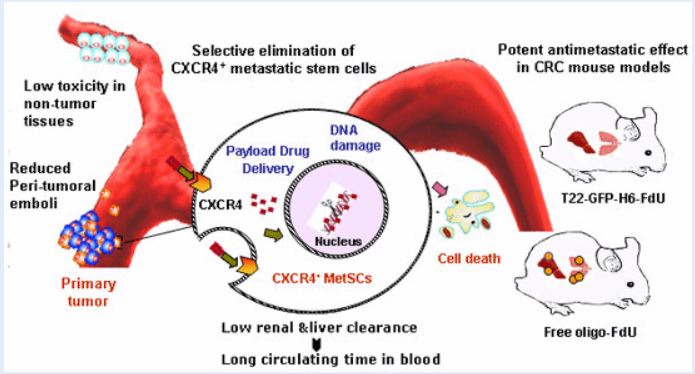
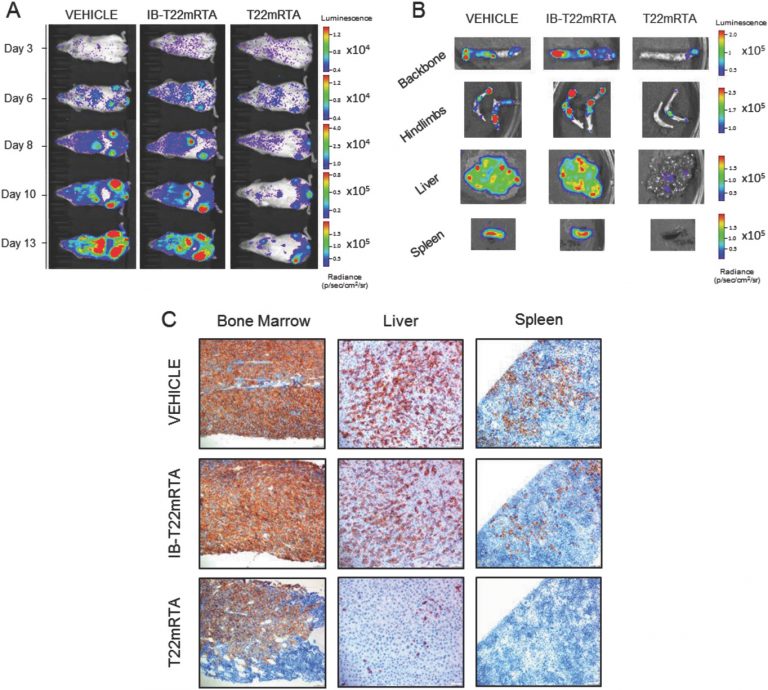
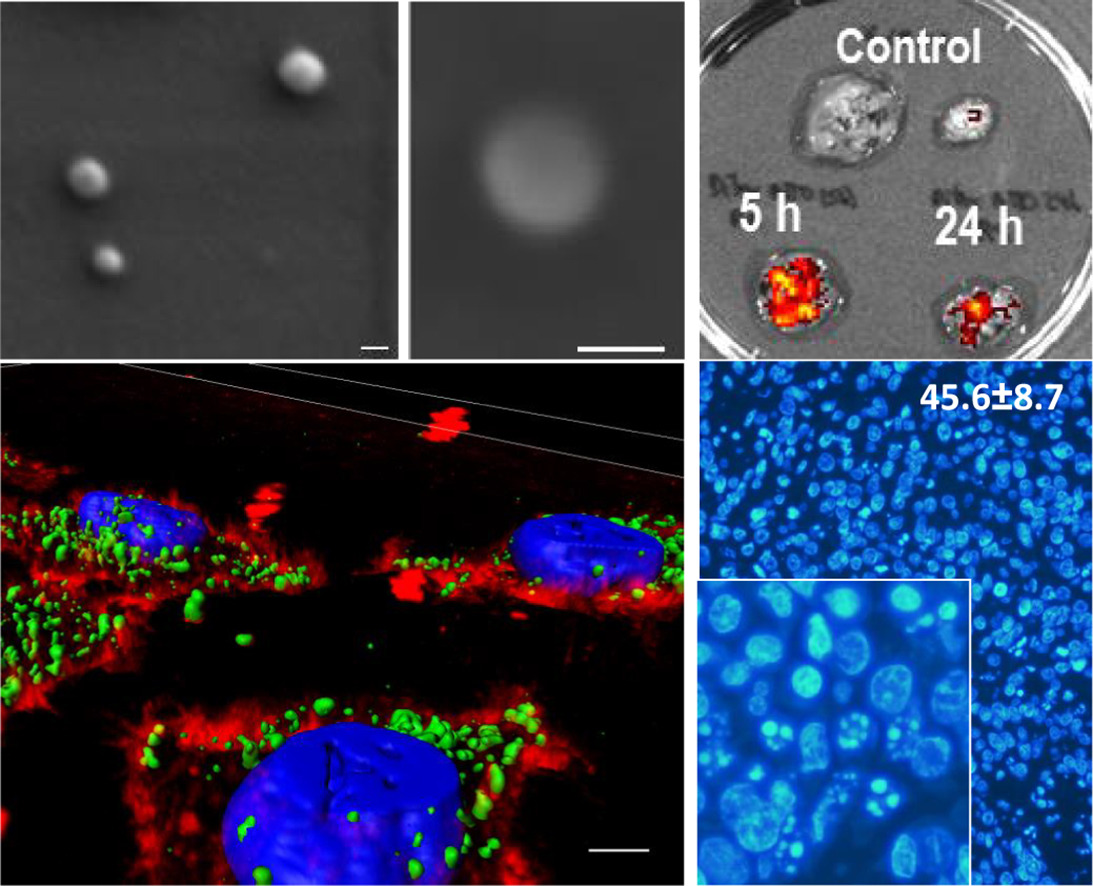
- Development of highly metastatic, CXCR4-overexpressing, colorectal cancer models. Rita Sala, Alberto Gallardo, Ugutz Unzueta, Patricia Álamo, Carmen Cabrera, Esperanza Medina, Isolda Casanova, Irene Arroyo, Aïda Falgás, Carlos Navas, Manuel Trías, Antonio Villaverde, Esther Vázquez, Ramón Mangues, Mª Virtudes Céspedes (GOA-HSPau) (U18)
- PATHGATE: Oligonucleotide-gated sensing nanodevices for pathogen detection. Luis Pla, Angela Ribes, M. Angeles Tormo-Mas, Javier Pemán, Félix Sancenón, Elena Aznar, Ramón Martínez-Máñez and Sara Santiago-Felipe (IQMA-IDM-UPV) (U26)
- Towards chemical communication between abiotic nanoparticles and living systems. Beatriz de Luis, Paola Ricón, Cristina de la Torre, Antoni LLopis, Jose Gadea, Jose R. Murguía, Félix Sancenón, Ramón Martínez-Máñez, Elena Aznar (IQMA-IDM-UPV) (U26)
- Development and in vitro evaluation of antimicrobial polymer coatings for the prophylactic treatment of hernia repair materials. Bárbara Pérez-Köhler, Selma Benito, Mar Fernández-Gutiérrez, Gemma Pascual, Marta Rodríguez, Verónica Gómez-Gil, Francisca García-Moreno, Julio San Román, Juan Manuel Bellón (GITBIT-UAH) (U17)
- Experimental study of the application of a new bone cement loaded with broad spectrum antibiotics for the treatment of bone infection. Joaquín García, Galo Azuara, Blanca Ibarra, Miguel A. Ortega, Ángel Asúnsolo, Blanca Vázquez, Julio San Román, Julia Buján, Basilio De la Torre, Natalio García-Honduvilla (GITBIT-UAH) (U17)