“Smart-4-Fabry”: European project focused on the Fabry rare disease, participated by 4 units of NANBIOSIS
- Smart-4-Fabry is a project coordinated by CIBER-BBN, funded by the European Commission within the Horizon 2020 Research and Innovation program with € 5.8 M for 4 years, which aims to develop a new nanomedicine for the treatment of the Fabry rare disease.
- Fabry disease is a rare disease belonging to the group of lysosomal storage disorders, with a global incidence of 1:5,000 – 1:10,000, representing a priority health problem at European level.
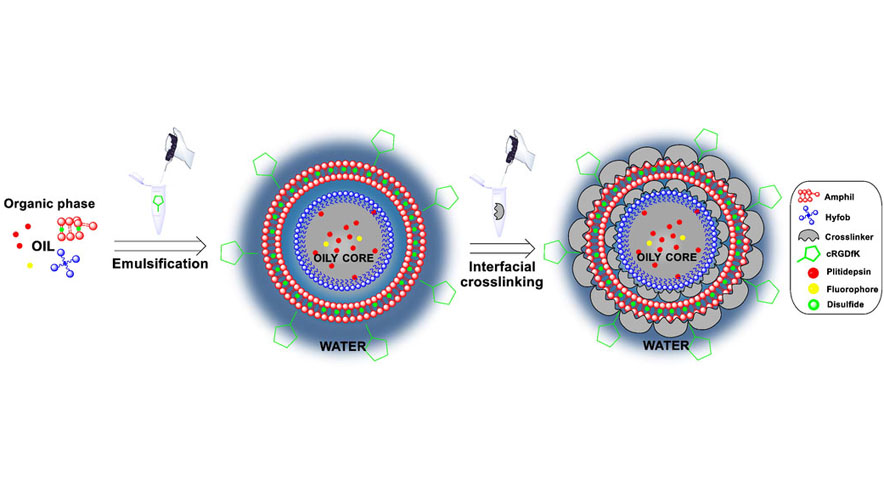
The European project “Smart-4-Fabry”, is coordinated by CIBER-BBN, specifically by NANOMOL group at ICMAB-CSIC (Dr. Nora Ventosa) and the Biomaterial Processing and Nanostructuring Unit (U6) of ICTS “NANBIOSIS”, and it also counts with the participation of NANBIOSIS Units U1 Protein Production Platform (PPP), U3 Synthesis of Peptides Unit, and U20 In vivo Experimental Platform.
Fabry disease is an inherited genetic disorder of the lysosomal storage group, which affects many organs and parts of the body, as it is caused by the accumulation of a lipid in the lysosomes of the cells, altering their functions and leading to cell death. This accumulation is due to the lack of an enzyme, α-Galactosidase A (GLA). The symptoms are many: limb pains, stains on the skin, problems with sweating, blurred frontal vision, gastrointestinal problems, loss of hearing, etc. In the long term it can cause renal failure, and heart and central nervous system problems.
Patients can lead a normal life with the current treatment called “enzyme replacement therapy”, where GLA is administered intravenously to patients. However, this treatment exhibits several drawbacks, related to a high instability, high immunogenicity or low efficacy of this molecule crossing cell walls. The development of a new treatment for this disease, as well as for other rare diseases, has become a priority challenge within the European program H2020.
Smart-4-Fabry, acronym for “Smart functional GLA-nanoformulation for Fabry disease”, was born with the idea of obtaining a new nanoformulation of GLA that will improve the efficacy and tolerance of the existing treatments. The project will advance from experimental proof of concept, to the preclinical regulatory phase. The ultimate goal is to reduce the treatment cost and to improve the quality of life of patients with Fabry disease.
Smart-4-Fabry, involves the participation of fourteen partners from five different countries from academia and industry. The consortium is formed by: Network of Biomedical Research Centers: Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) with the NANOMOL group at the Institute of Materials Science of Barcelona (ICMAB-CSIC), the Drug Delivery and Targeting Group at the Vall d’Hebron Research Institute (GDLF-VHIR), the Peptide Synthesis Unit at the Barcelona Science Park (UQC-PCB), and the Biotechnology and Biomedicine Institute of the Autonomous University of Barcelona (IBB-UAB) (Spain); Aarhus University (Denmark); Technion Israel Institute of Technology (Israel); Joanneum Research (Austria); Biopraxis Research AIE (Spain); the spin off Nanomol Technologies SL (Spain); BioNanoNet (Austria), Drug Development and Regulation SL (Spain), the Covance Laboratories LTD (UK) group; and Leanbio SL (Spain).
For further information: http://smart4fabry.eu/