Understanding Zeta Potential: Surface Charge at the Solid/Water Interface and Its Role in Modern Materials Science
Explore the importance of zeta potential and surface charge at the solid/liquid interface for biomaterials, membranes, and nanomaterials.
What is Zeta Potential and why does it matter?
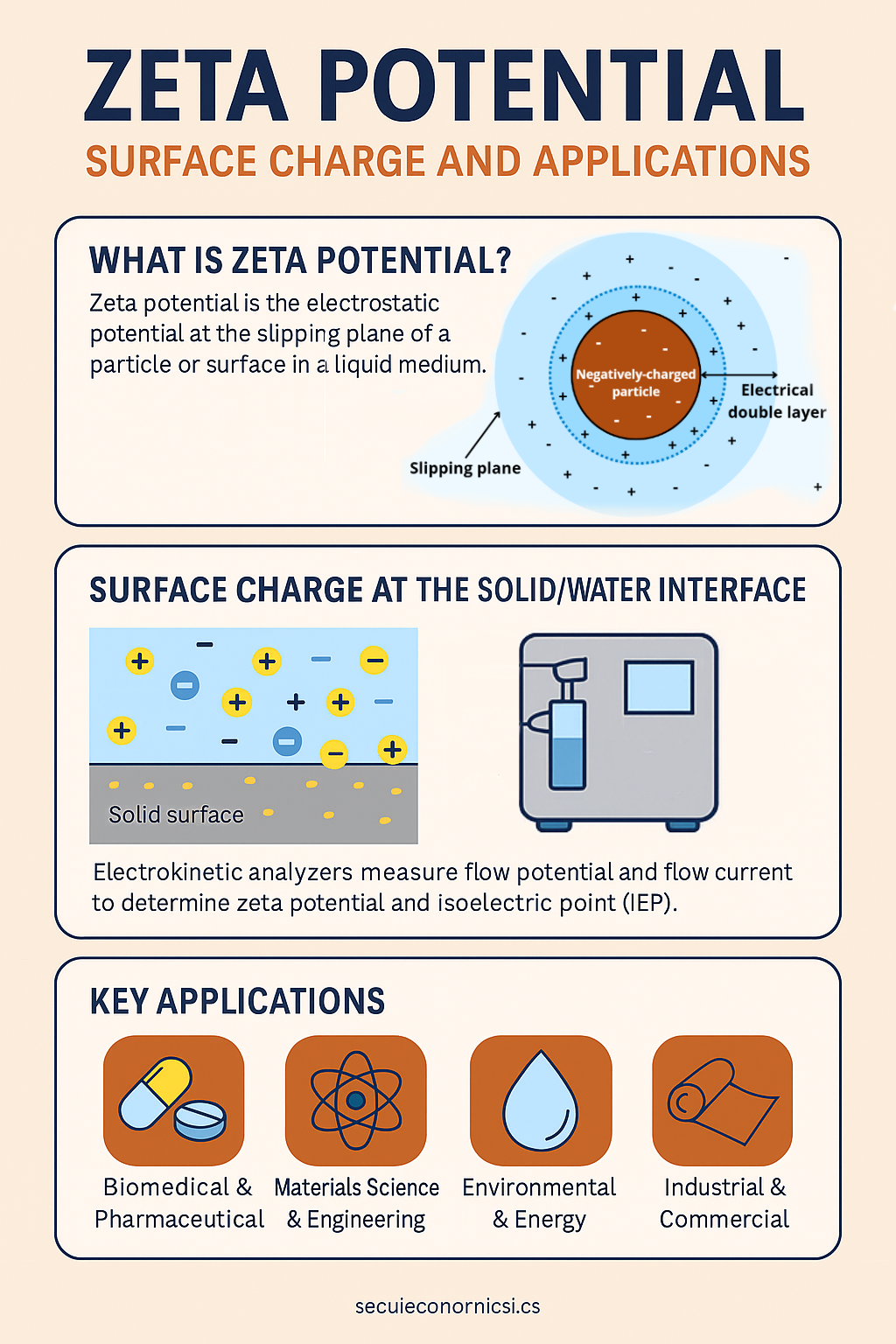
Zeta potential is a key physicochemical parameter that describes the electrostatic potential at the slipping plane of a solid surface in a liquid medium. It is not a direct measure of surface charge but rather the potential at the boundary between the stationary layer of fluid attached to the surface and the mobile layer of the surrounding liquid. This parameter is crucial for understanding the behavior of colloidal dispersions, emulsions, and particles in suspension.
The phenomenon of zeta potential emerges from the formation of the electrical double layer (EDL) at the interface between a solid and an aqueous solution. This layer consists of a charged surface and a compensating layer of counter-ions. When an external field is applied, the movement of these ions relative to the surface creates an observable potential difference.
Zeta potential impacts the stability of colloidal systems: high absolute values (positive or negative) indicate strong electrostatic repulsion, which helps prevent aggregation. Conversely, low values may signal a risk of flocculation or sedimentation. Hence, it is a critical metric in formulating stable suspensions in pharmaceuticals, cosmetics, food products, and beyond.
Surface charge at the solid/water interface
The solid/water interface is a dynamic region where charge develops due to several mechanisms: ionization of surface groups, ion adsorption, and lattice defects. The type and density of surface charge depend strongly on pH, ionic strength, and the nature of the surrounding electrolyte.
This surface charge is the origin of the electrical double layer and directly influences interactions with dissolved molecules, proteins, or ions. In biological and environmental systems, it governs key processes such as adsorption, desorption, ion exchange, and membrane transport.
In materials science, understanding surface charge is essential for tailoring materials with desired wettability, adhesion, or biocompatibility. This is especially relevant in applications involving membranes, coatings, and nanostructures that operate in aqueous environments.
How Zeta Potential is measured: techniques and technologies
Several techniques are used to determine zeta potential, including electrophoretic light scattering (ELS) for colloidal systems and streaming potential or streaming current methods for solid surfaces. Among advanced tools, the SurPASS 3 Electrokinetic Analyzer stands out for its ability to directly measure the zeta potential at the solid/liquid interface.
SurPASS 3 uses the classical electrokinetic approach with continuous flow: an electrolyte is passed through a channel formed between the sample surface and a reference, and the resulting flow potential or flow current is measured. This allows for precise, non-destructive analysis of a wide variety of sample geometries, including flat surfaces, powders, fibers, and porous materials.
Moreover, SurPASS 3 integrates automated pH titration using syringe pumps, enabling the determination of the isoelectric point (IEP). This is invaluable for tracking surface modifications and understanding material behavior across different pH levels. This equipment is available in the services of our Unit 16, among other surface characterization techniques.
Key applications across industries
Biomedical and Pharmaceutical
- Implants: Evaluation of surface charge helps optimize biocompatibility and reduce immune rejection.
- Drug delivery: Zeta potential measurements inform the design of nanoparticle carriers to enhance targeting and stability.
- Contact lenses: Assessment of protein adsorption through surface charge analysis supports development of more comfortable and hygienic lenses.
Materials science and engineering
- Membrane characterization: Understanding surface charge assists in improving antifouling properties and selectivity.
- Nanomaterial design: Enables engineering of coatings like graphene oxide with specific interfacial behaviors.
- Coating and adhesion studies: Surface charge insights guide the functionalization and durability of advanced materials.
Environmental and energy applications
- Fuel cell membranes: Characterizing zeta potential supports optimization of ion transport layers.
- Water purification: Adsorbent and filter materials benefit from surface charge tuning for enhanced contaminant removal.
Industrial and commercial uses
- Textile finishing: Zeta analysis supports better dyeing, treatment, and functional coatings.
- Food packaging: Helps in developing antimicrobial or oxygen-barrier films.
- Construction materials: Surface property evaluation leads to more durable and weather-resistant materials.
Competitive edge of SurPASS 3 vs other equipment
Compared to traditional surface analysis equipment, SurPASS 3 offers:
- Automation: Rapid, reproducible results with minimal user intervention.
- Versatility: Accommodates diverse sample shapes and sizes.
- pH-dependent profiling: Automatically determines IEP and adsorption/desorption kinetics.
- Real-time monitoring: Enables observation of surface transformations during chemical treatments.
However, barriers exist:
- Sample requirements: Specific geometries and physical properties are needed.
- Infrastructure needs: Compressed nitrogen supply and optional temperature control increase setup costs.
- Technical expertise: Trained operators are essential for accurate interpretation and maintenance.
Future outlook: emerging and visionary applications
In the near term, SurPASS 3 will continue supporting:
- Real-time adsorption studies for R&D
- Surface engineering of biomaterials
- Environmental material design (e.g., photocatalysts, adsorbents)
Long-term applications include:
- 4D-printed responsive materials with programmed zeta profiles
- Nanomaterials for quantum devices with controlled interfacial properties
- Virus-trapping smart surfaces for healthcare settings
- Carbon capture materials using charge-optimized MOFs
Final thoughts: why Zeta Potential is a foundational metric
Zeta potential is not just a measurement—it’s a gateway to understanding how materials behave at the most fundamental level. From drug delivery to environmental technology, from textile innovation to nanotechnology, the surface charge at the solid/liquid interface defines interactions, stability, and performance.
With tools like the SurPASS 3, researchers and engineers can now explore these properties with unmatched precision and adaptability, paving the way for smarter, more functional materials.
Credits:
Margarita Hierro Oliva
Gabriel Alfranca
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: