Good News! Protein Nanoparticles with a New Ligand Select and Destroy Tumor-associated Fibroblasts”
With the participation of two units of NANBIOSIS ICTS and the expertise of the scientists managing these units
The study, fruit of the collaboration between the Nanotechnology group of the Institute of Biotechnology and Biomedicine (IBB-UAB), led by Prof. Antonio Villaverde, and the Oncogenesis and Antitumor Drugs group of the Sant Pau Research Institute, led by Dr. Ramon Mangues, both members of CIBER-BBN, has made significant progress by identifying the natural ligand PDGFD as an effective tool to target protein nanoparticles to tumor-associated fibroblasts that overexpress the PDGFR-β receptor. Given the relevance of the discovery, this technology has been intellectually protected by a patent that is currently being processed (PCT/EP2023/081937).
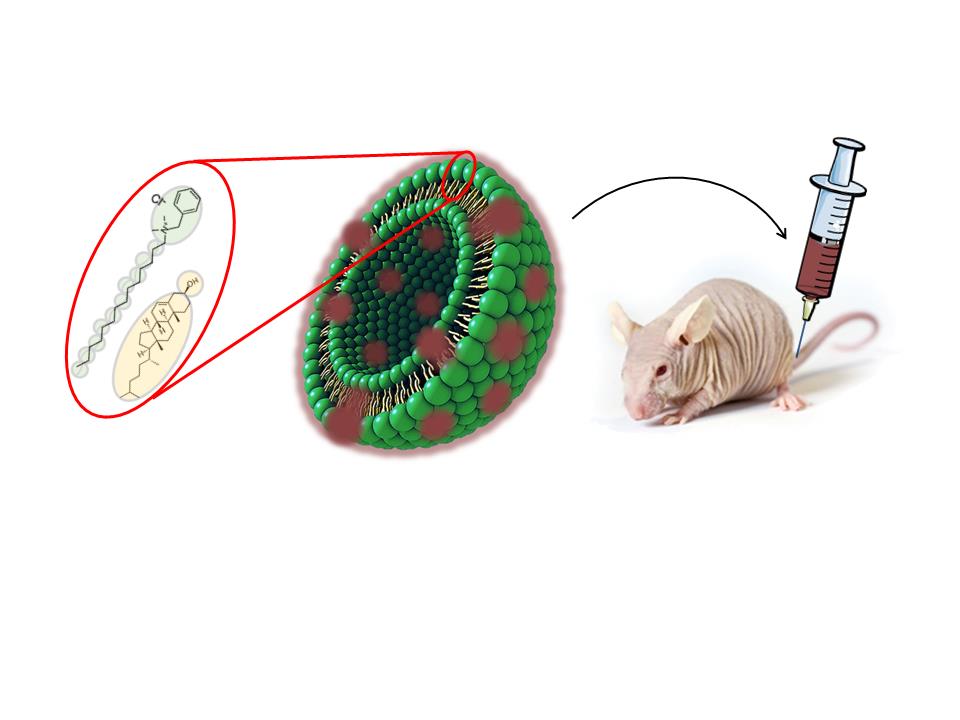
The research, the details of which have recently been published in the journal Acta Biomaterialia, presents an innovative strategy focused on the development of protein nanoparticles that assemble autonomously and are capable of selectively recognizing and destroying tumor-associated fibroblasts with high levels of PDGFR-β. This cell type plays a fundamental role in the tumor microenvironment, providing mechanical and biological support for tumor growth and progression in various types of cancers.
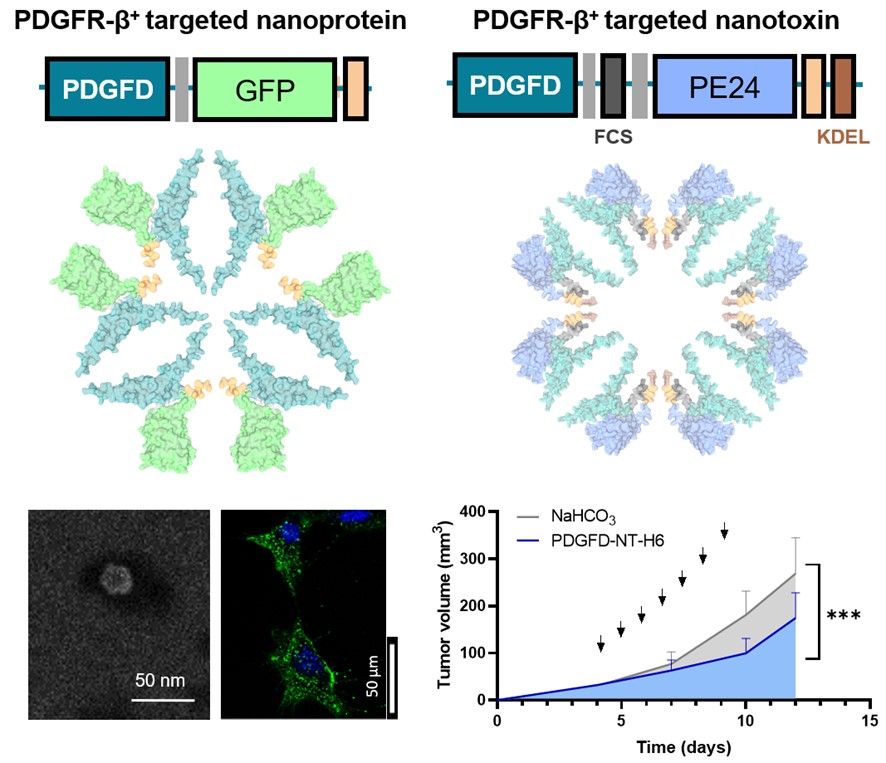
Taking advantage of their solid experience in the development of tumor-targeting protein nanoparticles and their functional characterization in in vitro and in vivo models of different types of cancer, both groups set out on this occasion to design new nanoparticles targeting tumor-associated fibroblasts with PDGFR-β overexpression. Among the different ligands tested, PDGFD has been selected for its ability to induce selective penetration into target cells both in vitro and in vivo, using a murine model with a subcutaneous tumor. In these experiments, the PDGFD-GFP-H6 fusion protein, formed by the chosen ligand, the green fluorescent protein and a histidine tail with an important role in obtaining nanoparticles, accumulates precisely in tumor tissues, demonstrating its ability from being delivered in tumor.
By replacing GFP with a microbial toxin present in antitumor treatments approved for clinical use, a significant reduction in tumor volume growth is observed, without showing toxic collateral effects in mice. In this way, the PDGFR-β/PDGFD couple has been validated as a versatile tool for the targeted delivery of drugs to the tumor microenvironment. These promising results pave the way for future developments in nanomedicine and offer new hope in the search for more effective and less invasive treatments for cancer patients.
The research has been performed with the collaborative participation of two units of the ICTS “NANBIOSIS”, more specifically the units U1 of Protein Production Platform, PPP and U18, Nanotoxicology Unit, and is framed in the context of the intramural collaboration of the CIBER-BBN “FIBOLISM”, coordinated by Dr Lorena Alba Castellon.
Referenced article
Eric Voltà-Durán•, Lorena Alba-Castellón• , Naroa Serna, Isolda Casanova, Hèctor López-Laguna, Alberto Gallardo, Alejandro Sánchez-Chardi, Antonio Villaverde, Ugutz Unzueta, Esther Vázquez, Ramón Mangues*. High-precision targeting and destruction of cancer-associated PDGFR-β+ stromal fibroblasts through self-assembling, protein-only nanoparticles. Acta Biomaterialia 170 543-555 (2023) https://doi.org/10.1016/j.actbio.2023.09.001
• Equal contribution
*Corresponding authors