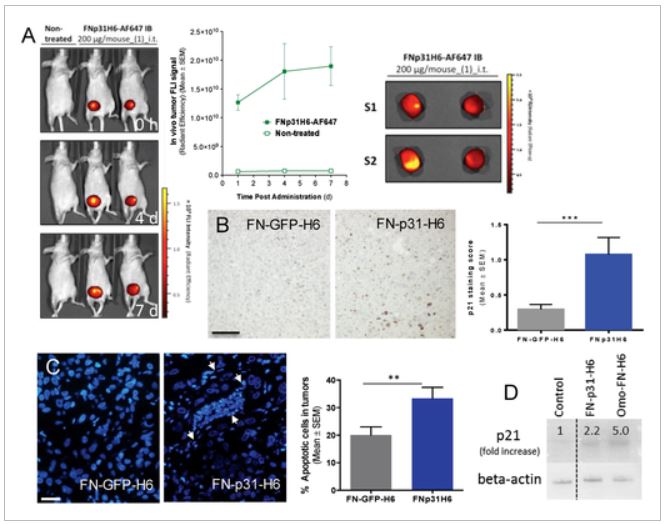
Targeting of the breast cancer stem cells to improve the treatment of triple negative breast cancer
On April 1st PhD candidate Patricia Cámara Sánchez defended her doctoral thesis entitled “Targeting of the breast cancer stem cells to improve the treatment of triple negative breast cancer“, where ICTS-Nanbiosis Unit 20 participated in the in vivo assays. The thesis was supervised by Dr. Ibane Abasolo (scientific director of Unit 20 from CIBER-BBN and VHIR). Nanbiosis was also present within the jury, with Dr. Ana Paula Candiota (scientific coordinator of Unit 25 from CIBER-BBN and UAB) acting as secretary.
Patricia Cámara graduated as biochemist, did the master’s degree in Translational Biomedical Research from VHIR-UAB. Shortly after, started the PhD, which was aimed at improving the treatment of very deadly subtype of breast cancer by using different nanoformulations to specifically target the cancer stem cells. The now doctor Cámara-Sánchez screened up to 20 small drugs with anti-cancer stem cell activity, found synergistic ratios with conventional chemotherapeutic agents, and finally developed polymeric micelles encapsulating selected drugs. During the discussion of the dissertation, the need of additional in vivo assays was highlighted, as well as the potential use of MR provided by the U25, to explore non-invasively the metabolomic differences between cancer stem cells and regular cancer cells.
‘I’m very grateful for the opportunity to have been part of this amazing project. It has been a very enriching experience’, she explains. ‘After 5 years of research, I finish the PhD feeling very proud of having contributed to the fight against this aggressive subtype of breast cancer’, she adds. From now on, new research lines will bring forward the synergies between both units of NANBIOSIS, reinforcing a collaboration started several years ago and reflected in joint papers.