A GLOBAL VISION OF THE COVID-19 PANDEMIC by CSIC
A report from the Spanish National Research Council (CSIC) collects in open access the results of a year of research on SARS-CoV-2. The book ‘A global vision of the COVID-19 pandemic’ shows the responses and solutions obtained by the 300 research teams of the CSIC Global Health Platform. The Institute for Advanced Chemistry of Catalonia (IQAC-CSIC) has participated in the preparation of the chapter ‘Actions in containment and diagnosis’:
- Specific mucosal protection against the entry of SARS-CoV-2 – Lluisa Coderch.
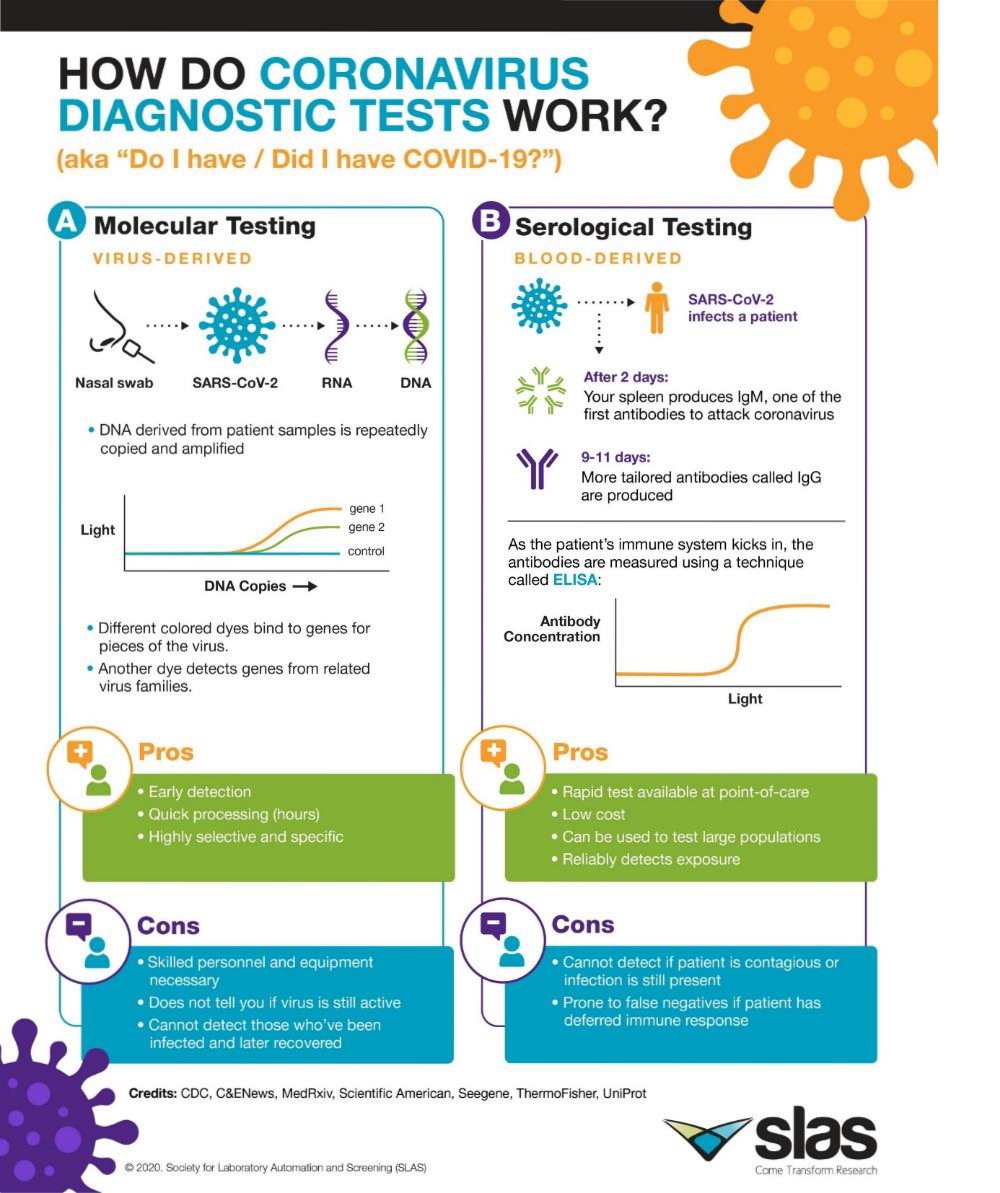
- The role of diagnosis in the face of the pandemic – by M.-Pilar Marco, Scientific Director of NANBIOSIS U2 Custom Antibody Service (CAbS)
Visit CSIC website to read the full news.