New look of Nb4D – CAbS (NANBIOSIS U2) “Revolutionising Diagnosis”
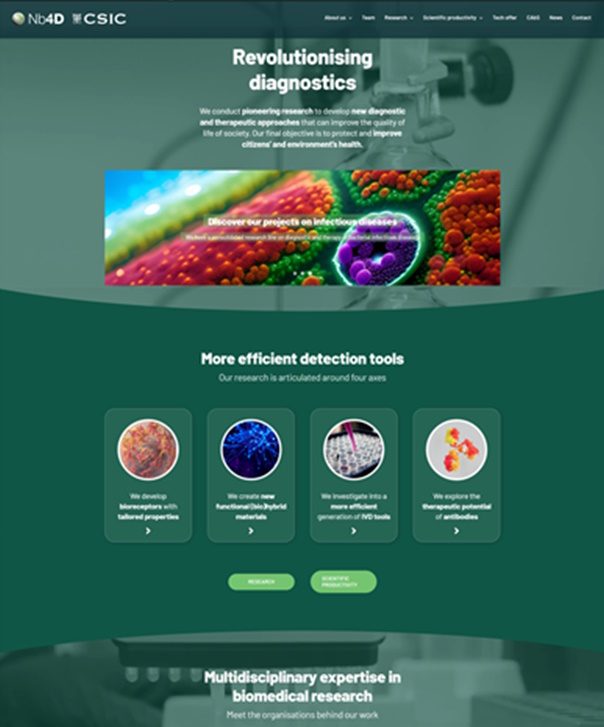
Nb4D has a new look on line! Nb4D Group (of IQAC-CSIC and CIBER-BBN) has lunched a new website “Revolutionsising Diagnosis“ with the aim to facilitate a faster and easier navigation througth their “pioneering research to develop new diagnostic and therapeutic approaches” and their solutions and expertise to help researchers and companies.
Antibodies, bioreceptors, hapten design and synthesis, immunoanalytical method development, new ivd tools, surface functionalization, therapeutic antibodies and much more knowledge and expertise revolutionising diagnosis.
The new website contains a page for CAbS-NANBIOSIS. Custom Antibody Service (CAbS), unit 2 of the ICTS NANBIOSIS