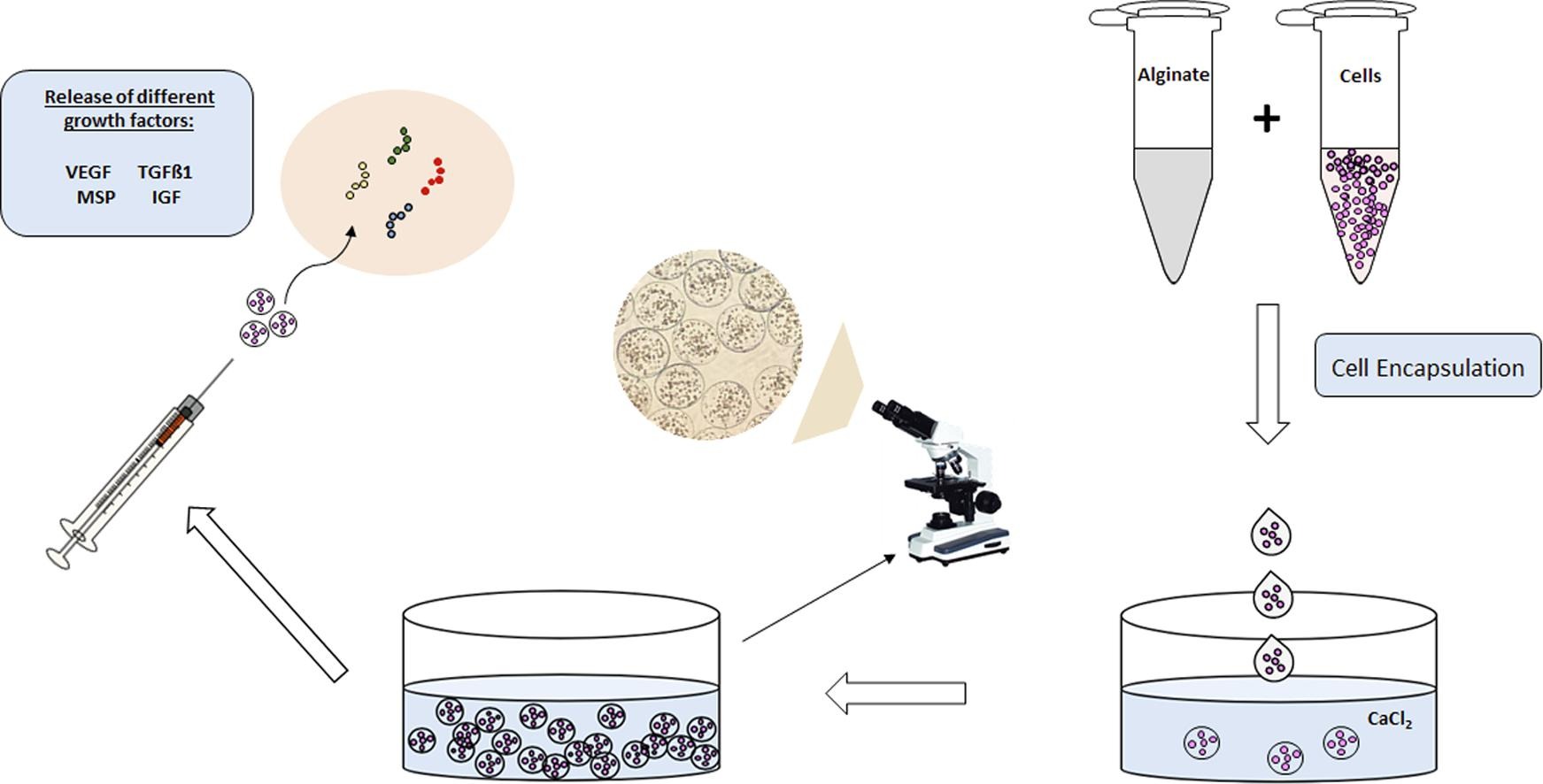
Characterization of encapsulated porcine cardiosphere-derived cells for cardiac regeneration.
Researchers of NANBIOSIS U10 Drug Formulation unit of CIBER-BBN and UPV/EHU and NANBIOSIS U14 Cell Therapy unit and U24 Medical imaging unit at CCMIJU have participated in a research that proposes the use of encapsulated cardiosphere-derived cells (CDC), obtained from heart tissue, as regenerative cell therapy.
“The encapsulation of the cells has been carried out in NANBIOSIS Unit 10, as well as the characterization of the cells (both encapsulated and unencapsulated) for their application in regenerative medicine (cardiac regeneration); and unit 14 of NANBIOSIS has obtained the cell model used for the study “ – Explains Kaoutar Ziani Akrirout, research scientists of CIBER-BBN and NANBIOSIS Unit 10.
These cells are multipotent stem cells, which secrete growth factors capable of promoting revascularization and healing of infarcted tissue. However, the use of this therapy faces a great challenge, which is the survival and retention of these cells after their implantation in the infarcted area, since the heart is a tissue that is constantly contracting and expanding, which leads to the loss of these cells, as they are carried along by the bloodstream.
To solve the low retention of cells, members of the NanoBioCel research team, from the CIBER-BBN and attached to the Bioaraba Health Research Institute (IIS Bioaraba), in collaboration with researchers from the IIS Aragón and the CIBERCV, propose the encapsulation of the CDC of porcine origin within a three-dimensional alginate-poly-L-lysine-alginate matrix as a therapy for cardiac regeneration, since, thanks to this, the encapsulated cells will be able to remain adhered to the tissue for longer, giving them time to exercise their function. The final objective will be to verify its efficacy in swine infarction models.
The team has verified that the phenotypic characteristics, the gene expression profile, the ability to differentiate to other cell lines and the release of growth factors from these cells are not altered by the encapsulation process, essential aspects given that their preservation it is essential for cardiac regeneration. In addition, this procedure keeps them viable for a month, which would favor the possible regeneration of the tissue.
On the other hand, that a sustained release of growth factors is maintained in these cells suggests that the implantation of encapsulated CDCs will promote the formation of new blood vessels and, consequently, the regeneration of infarcted cardiac tissue.
The researchers suggest that encapsulated CDCs could be a highly interesting therapeutic alternative in the field of cardiac regenerative medicine.
Article of reference
Ziani K, Espona-Noguera A, Crisóstomo V, Casado JG, Sanchez-Margallo FM, Saenz-Del-Burgo L, Ciriza J, Pedraz JL. Characterization of encapsulated porcine cardiosphere-derived cells embedded in 3D alginate matrices. Int J Pharm. 2021 Apr 15;599:120454.[DOI]