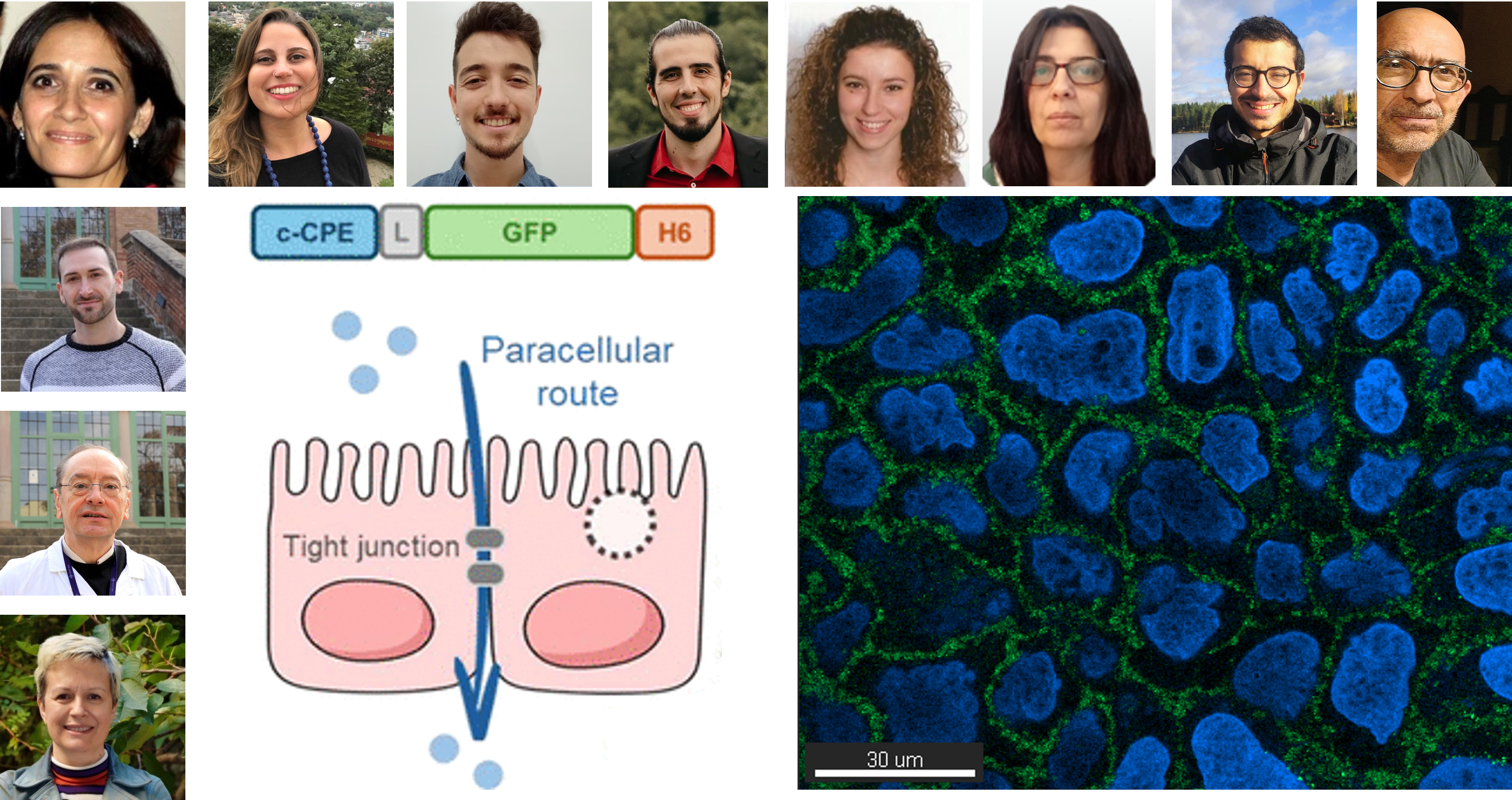
Researchers develop a promising therapy for alcohol‑induced brain injury thanks to NANBIOSIS services
First study using NANBIOSIS Unit 26 PET/MRI‑3T shows MSC-EVs reduce alcohol-induced brain damage and neuroinflammation in female mice.
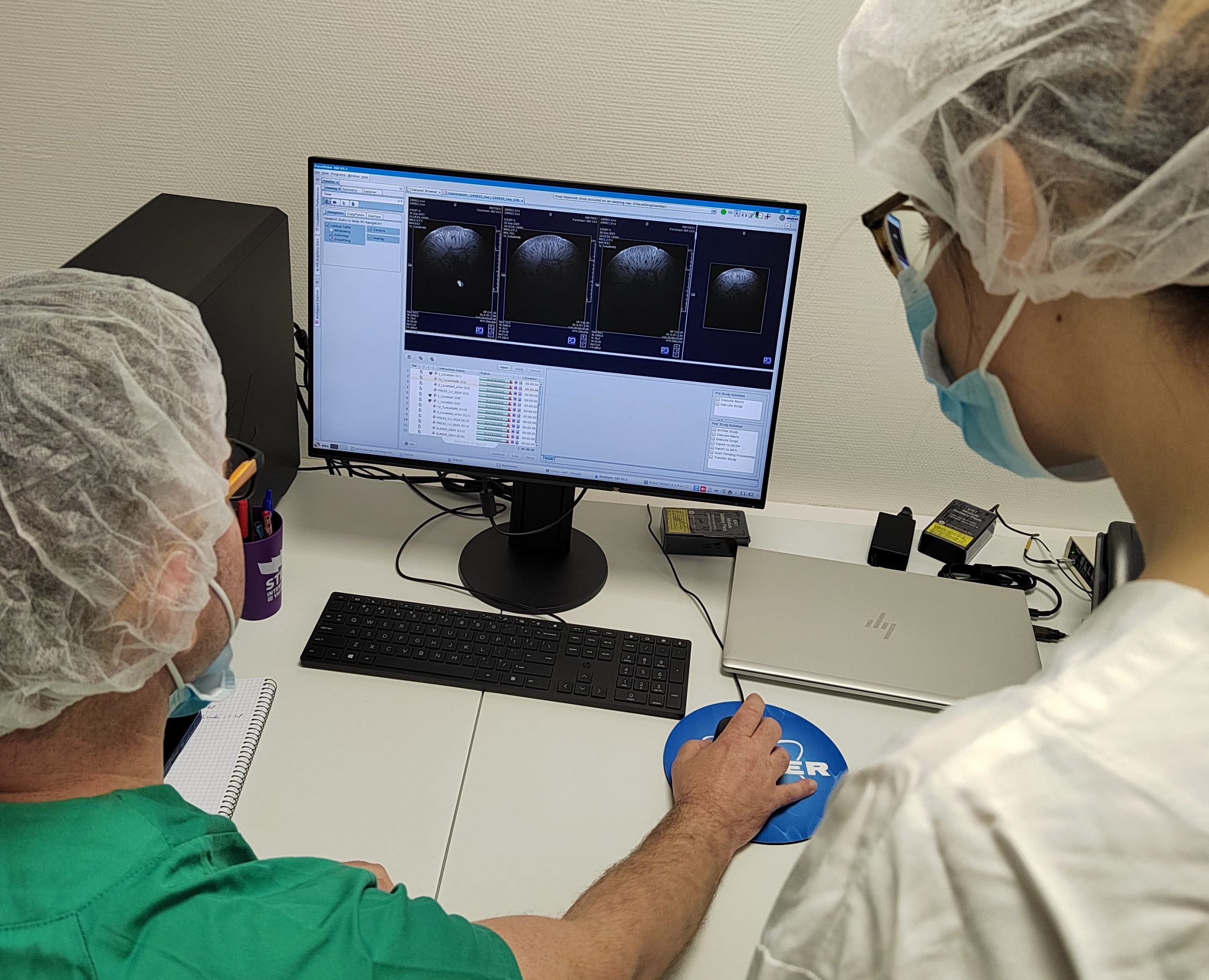
Valencia, Spain – The Spanish national infrastructure NANBIOSIS is celebrating a significant breakthrough: the first scientific article utilizing PET/MRI‑3T imaging services from Unit 26 has been published, showcasing a novel therapy against alcohol-induced brain damage in female mice.
Study overview & key findings
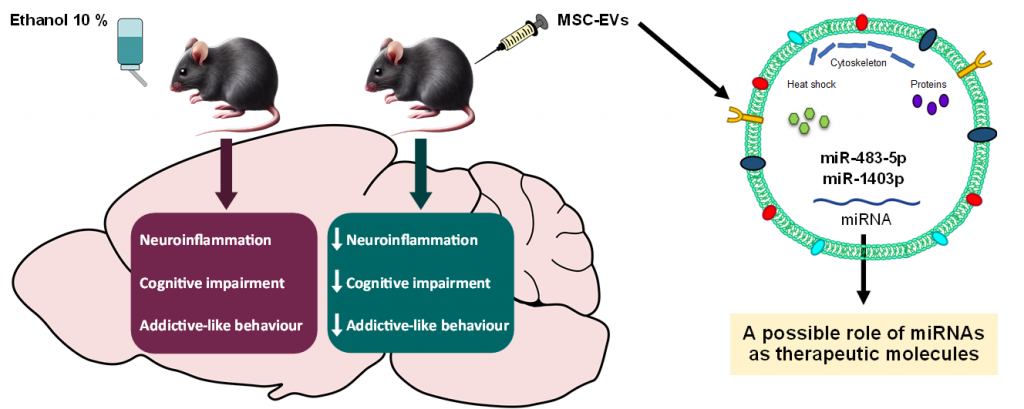
The article, titled “Role of miRNAs from mesenchymal stem cell‑derived extracellular vesicles in neuroinflammation and behavioral impairments induced by chronic alcohol consumption in female mice“, appeared online ahead of print in Neural Regeneration Research on June 19, 2025 . It demonstrates that repeated intravenous doses of mesenchymal stem cell-derived extracellular vesicles (MSC‑EVs), rich in regulatory miRNAs, effectively:
- Reduced alcoholism-driven neuroinflammation, confirmed by lower expression of pro-inflammatory genes like Tnf, Il1b, Mtor and Atf6
- Improved cognitive behavior and attenuated sensitivity to cocaine’s conditioned reward
- Decreased abnormal brain glucose metabolism, shown via reduced uptake of the PET tracer ^18F-FDG
Role of PET/MRI‑3T Imaging from NANBIOSIS
This milestone became possible thanks to the recently launched PET/MRI‑3T platform of Unit 26: a state-of-the-art preclinical imaging system combining a 3 Tesla MRI with integrated PET capabilities. This dual-modality setup enables precise metabolic and anatomical visualization in vivo, allowing researchers to perform longitudinal studies in small rodents. In this case, the imaging revealed a reversal of ethanol-induced metabolic dysregulation in the brains of treated mice.
The relevance of these findings
These results provide compelling in vivo evidence that miRNA-enriched MSC-EVs can counteract alcohol-induced brain damage. In addition, it etablishes NANBIOSIS Unit 26 as a leader in multimodal preclinical imaging, bridging molecular insights with live metabolic data. Finally, this research opens new avenues for research in neuroscience, regenerative medicine, and addiction therapy.
About NANBIOSIS Unit 26
Housed at the Faculty of Medicine at University of Valencia, NANBIOSIS Unit 26 is led by Dr. Ramón Martínez Máñez (UPV) and Dr. Salvador Gil (UV-SCSIE). Beyond the PET/MRI‑3T, the Unit offers, among other services, a 14 Tesla NMR spectrometer for ex vivo metabolic profiling and advanced support for in vivo and ex vivo metabolic and molecular imaging in rodents.
Strategic impact and future directions
This publication not only highlights the scientific value of MSC-EV therapy for alcohol-related neuroinflammation but also spotlights NANBIOSIS imaging capabilities. Future research leveraging this infrastructure could revolutionize fields such as neuropharmacology, regenerative medicine, and translational research, thanks to its ability to bridge preclinical data to human therapies.
Access the full article: see Neural Regeneration Research, June 19, 2025, doi:10.4103/NRR.NRR‑D‑24‑01260
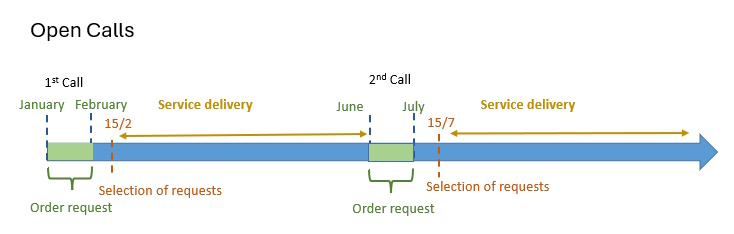
And do not forget that our last Open Call of 2025 is open until June 30. Visit here for more information.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: