The Medicine of the Future needs the Nanomedicine Revolution. This is why
The medicine of the future is an increasingly tackled topic. In the context of global concern for the sustainability of the health system (chronic diseases, new disorders, aging population and financing problems), nanomedicine could promote more affordable and personalized health care and improve the quality of life of the patients.
Between innovative techniques already implemented and concepts that evoke science fiction (nanobots, fluorescent particles working as spies, tiny Trojan horses introduced into our body …), nanomedicine generates great expectations.
Nanomedicine, what is it exactly?
Nanomedicine is the application of nanotechnology to medicine, that is, the use of nanotechnologic systems for the prevention, diagnosis or treatment of diseases, due to the particular properties that materials present on a nanometric scale. (Yes, although it seems strange, the same material has totally different attributes and behaviours when “nano” amounts of it are manipulated, what is very important in medicine, since many of the processes of the human body take place on a nanometric scale).
The current state, thanks to the previous effort.
When in 1959 Richard Feynmand, (Nobel Prize in Physics in 1965), gave his speech “There is a lot of space down there”, he opened the door to research at the nano scale: from 1nm to 100nm, this is one-millionth of a millimeter (10-9 meters); we are talking about the range of sizes resulting from dividing the diameter of a hair between 1,000 and 10,000, (or what a nail grows in a second).
Since the entry into the market of the first nanomedicine in 1995 (Doxil®, a drug encapsulated in liposomes for the treatment of cancer), nanoparticles or nanostructures have been developed for the controlled release of drugs in cancer and other pathologies, nanodevices have been created for disease diagnosis or nanomaterials have been designed for applications in regenerative medicine, and even messenger RNA vaccines for Covid-19, such as those from Pfizer and Moderna, are nanoformulated. Today there are on the market a hundred nanoformulated drugs all thanks to previous research and development of nanomaterials and nanoparticles over the last three decades.
The “Observatory of Trends in Medicine of the Future” promoted by the Roche Institute foundation has recently published a Report on Nanomedicine coordinated by Dr. Ramón Martínez Máñez, Professor of Inorganic Chemistry at the UPV and Scientific Director of the Centre for Networked Biomedical Research in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) in which Dr. José Becerra, Professor of Cell Biology at the University of Malaga and Principal Researcher at CIBER-BBN, BIONAND and IBIMA, Dr. Pilar Marco, Principal Investigator of the Nanobiotechnology group for the diagnosis (Nb4D) of the IQAC-CSIC and Coordinator of the Nanomedicine Research Program of the CIBER-BBN and Dr. María Jesús Vicent, Chief Researcher of the Therapeutic Polymers Laboratory and coordinator of the Advanced Therapies Area of the Príncipe Felipe Research Center have participated as experts. The report was presented at the IV Conference “Anticipating the Medicine of the Future” on November 30, 2021 where a debate was held by the above mentioned in which various topics related to nanomedicine were discussed, such as its applications and barriers.
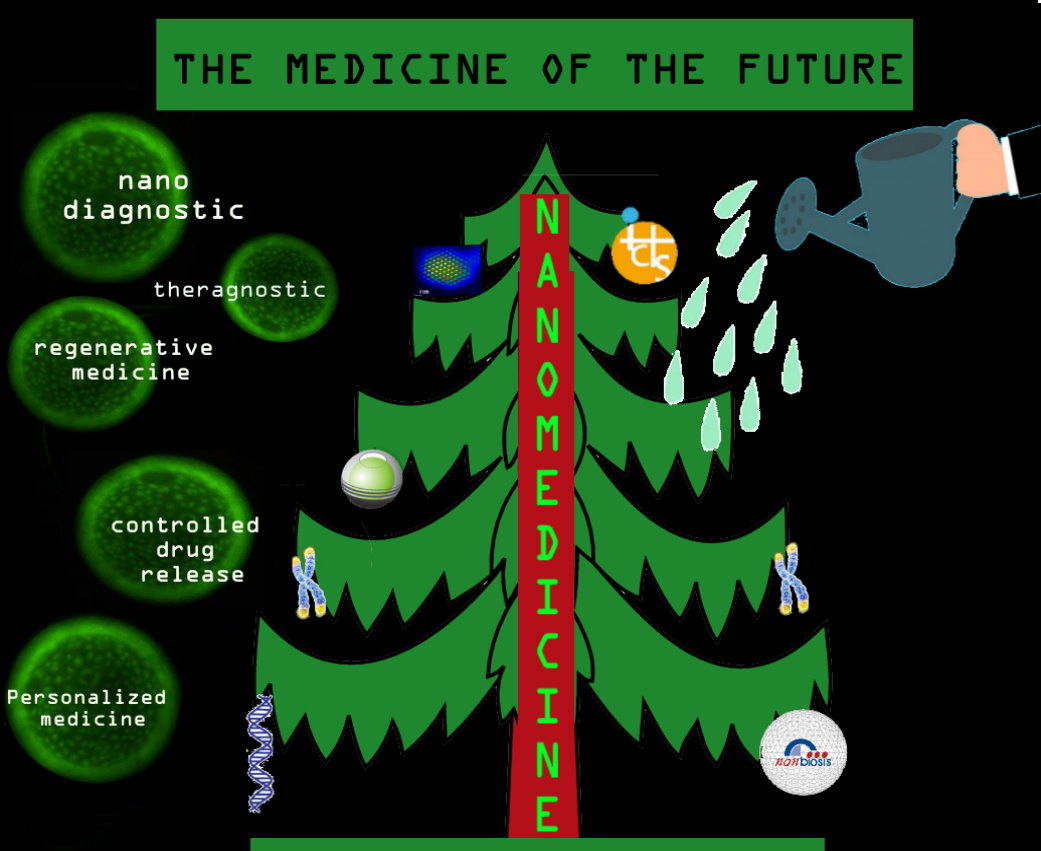
Nanomedicine applications of today and tomorrow
Nanomedicine is completely transversal, multidisciplinary and dependent on other disciplines, so its applications are multiple and complementary to other branches of knowledge such as artificial intelligence, but the following fields stand out fundamentally.
The design of nanomaterials that improve biocompatibility or biomechanical properties is investigated and can be used for the manufacture of implants that allow replacing portions of diseased tissue and that can even be designed in a personalized way attending to the individual response of each patient, minimizing the risk of rejection by the patient in regenerative medicine.
Nanoparticles are used to build highly sensitive nanodiagnostic platforms, which provide comprehensive biological information easily, quickly and economically at an increasingly early stage. Pilar Marco visualizes a future where “the diagnosis could be our molecular fingerprint, so that the detection of changes in said fingerprint could lead to the detection of a disease before the patient presents symptoms. In turn, this will contribute to prediction and prognosis since, if a large amount of information is available, it can be crossed with genetic information”.
Nanomedicine makes it possible to improve the pharmacokinetics and pharmacodynamics of current drugs, so that they specifically deploy their activity in diseased cells and tissues in a controlled way over time and crossing any biological barrier, which is called controlled drug release. According to Ramón Martínez “Any disease can be susceptible to use these systems to deliver a drug in the appropriate organ or tissue with the reduction of drug doses and side effects.”
Finally, nanotechnology methods facilitate the fusion of diagnosis and therapy in the new medical field of theragnostic; diagnose and treat at the same time by understanding the biological response to treatments, that is, the administration of drugs whose molecules allow visualize how the drug is working.
Barriers faced by nanomedicine
In addition to the difficulties presented by nanomedicine in matters of regulation and industrial property, the aforementioned experts agree that one of the most important challenges is the standardization of manufacturing procedures and quality controls, investment is needed in infrastructures to fine-tune manufacturing and standardization systems (manufacturing of nanoparticles under GMP) and in collaboration with the private sector, which is crucial, to make nanomedicine reach the productive sector and society.
But there are also barriers in the research itself, and funding is needed to break them down. In nanomedicine research, cost / effectiveness analyses have to be focused on the long term. Professor José Becerra explains it very clearly: “Research topics become fashionable and it happens frequently that the years go by and administrations “get tired” of financing a certain field and this is a problem because if a tree is planted by a person who knows It takes ten years to bear fruit, this person has to take care of the tree, but if we give the tree care to someone who does not know about trees, probably this person will abandon the tree in five years … Scientific policies have to persevere in financing nano and accompany it with an improvement in the regulation of products and only then will companies invest in this area”.
At the end of the debate, Professor José Becerra celebrated that the Carlos III Health Institute opted, fifteen years ago, for the creation of a CIBER in Bioengineering, Biomaterials and Nanomedicine, as a tool for scientific policy, he also mentioned the NANBIOSIS platform created by CIBER-BBN, CCMIJU and BIONAND, recognized as ICTS by the Ministry and available for companies and researchers to produce and characterize bio and nanomaterials, and stated that “it is evident that it is not possible to advance in the transfer of knowledgy from nano to the clinic at the same rate as is done in other knowledge areas but to take care of this project is essential”.
Related news:
Nanomedicine in the Medicine of the future
A more effective nanomedicine has been developed for the treatment of Fabry rare disease
Nanomedicine: how to get drugs to the place where they have to act.
A new generation of devices for the rapid, cheap and easy diagnosis of candidemia
New Nanomedicines for the topical treatment of complex wounds
Sources of information:
Nanomedicine (European Nanotecnology Platform)