New nanocarrier for bio-imaging and drug-delivery applications
Researchers of CIBER-BBN and NANBIOSIS-ICTS (U6 Biomaterial Processing and Nanostructuring Unit at ICMAB-CSIC and U18 Nanotoxicology Unit at Hospital de la Santa Creu i Sant Pau have developed a new nanocarrier for bio-imaging and drug-delivery applications
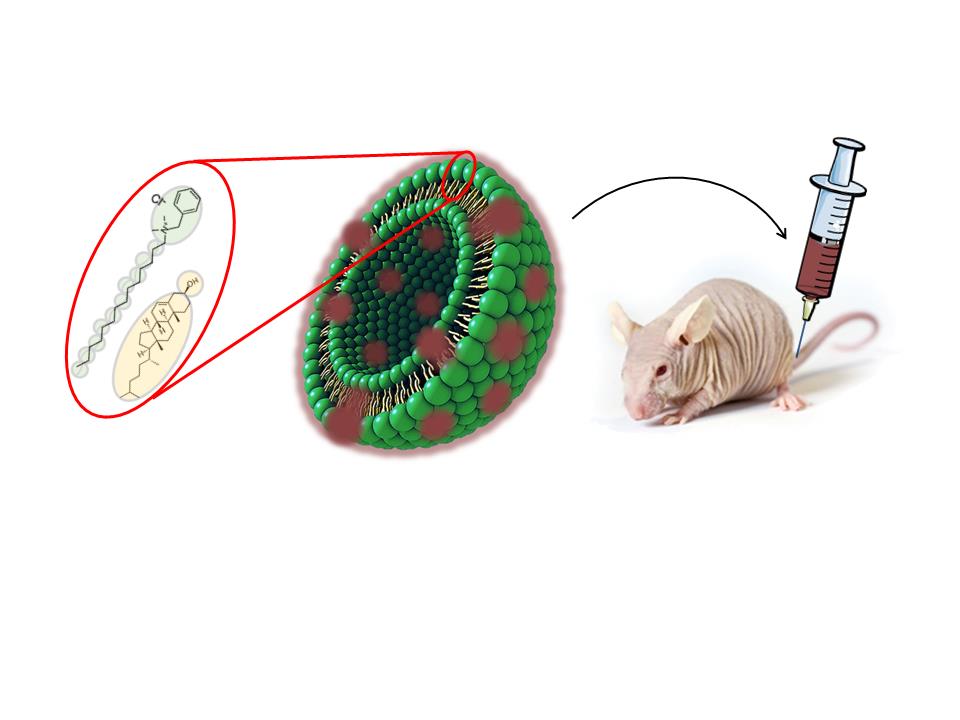
The new nanovesicle formulation is based on the quatsome architecture – which stands out due to the high colloidal stability and homogeneity in size – and has now been shown to be suitable for in vivo dosing.
Quatsomes are new non-liposomal lipid-based nanovesicles that have been developed by Nanomol group in recent years, and have been shown to be highly homogeneous and stable in different media for years. This colloidal stability involves important advantages for the development of pharmaceutical formulations and for guaranteeing the final product quality. Quatsomes are a promising nanocarrier for bio-imaging and drug-delivery applications, suitable for the encapsulation of both hydrophilic and hydrophobic molecules, easily functionalized with elements that favor the directionality towards therapeutic targets.
To facilitate their use in in vivo applications, Nanomol group has now developed a new Quatsome formulation, composed of cholesterol and myristalkonium chloride (MKC), the C14 homolog of benzalkonium chloride (BAK), the latter being extensively used as antimicrobial preservative in many ophthalmic and parenteral formulations on the EU and USA market. These novel MKC-Quatsomes have been synthesized in different media that are suitable for parenteral administration, in which they showed to be stable for at least 18 months. Moreover, vesicles remained stable in human serum for at least 24 hours.
In collaboration with the Oncogenesis and Antitumour Drug group of the Biomedical Research Institute of the Hospital de la Santa Creu i Sant Pau, these MKC-Quatsomes were tested in live mice bearing xenografted colorectal tumors. After intravenous injection of fluorescently labelled MKC-Quatsomes, biodistribution assays showed nanovesicle accumulation in tumors, liver, spleen, and kidneys, but not in any other organ. Importantly, MKC-Quatsomes were well-tolerated at the administered doses, and no histological alterations or toxicity was found in any of these organs. These new results suggest the applicability of quatsomes in therapeutic approaches that require systemic delivery.
NANOMOL group, Coordinator of NANBIOSIS U6 at ICMAB-CSIC and the Oncogenesis and Antitumor Drug group, coordinator NANBIOSIS U18 at Biomedical Research Institute (Hospital de la Santa Creu i Sant Pau) are members of Biomedical Research Networking center in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) and have a wide expertise and recognized excellence in the synthesis, processing and study of molecular and polymeric materials and the study of their biomedical properties. NANOMOL is also a member of the technology transfer network TECNIO. ‘
Article of reference:
MKC-Quatsomes. A stable nanovesicle platform for bio-imaging and drug-delivery applications co-authored by Guillem Vargas-Nadal et al., Nanomedicine: Nanotechnology, Biology and Medicine, 24 (2020) 102136. https://doi.org/10.1016/j.nano.2019.102136