Discover what HPLC is, how it works, and why it’s essential in analytical chemistry and biotechnology. Learn about real applications like LC/MSD iQ integration for antibody purification.
What is HPLC? Understanding High-Performance Liquid Chromatography
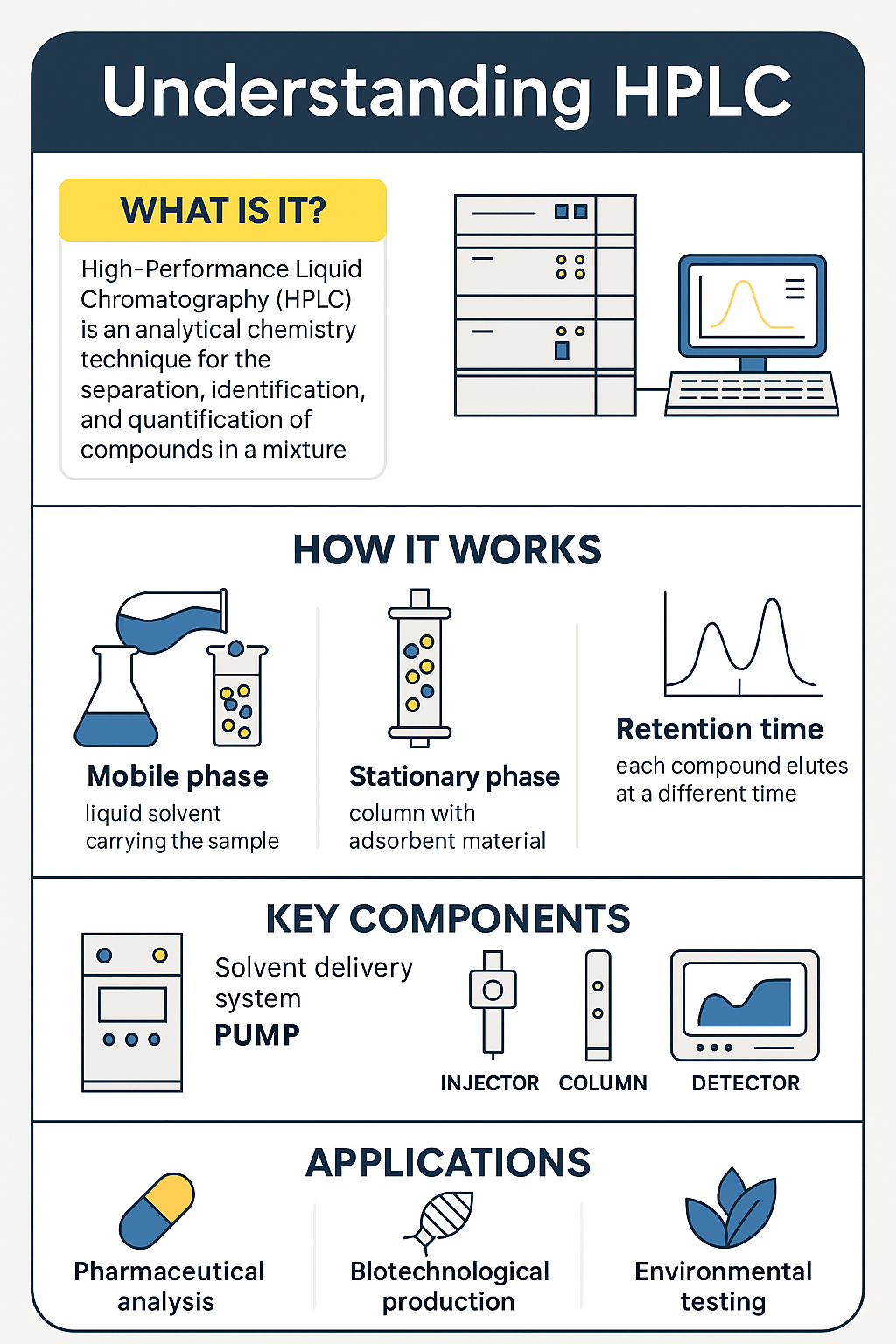
High-Performance Liquid Chromatography (HPLC) is one of the most powerful analytical techniques used in chemistry, biochemistry, and biotechnology. From pharmaceutical quality control to the purification of cutting-edge biotechnological products, HPLC provides high-resolution separation and precise quantification of complex mixtures. Its precision, sensitivity, and versatility have made it indispensable in both research and industrial settings.
This article explores how HPLC works, its key components, real-world applications —including the integration of mass detectors like the Agilent LC/MSD iQ— and how this technique is evolving toward automation and AI-enhanced scalability.
How does HPLC work? Core principles and mechanism
At its core, HPLC is a technique for separating, identifying, and quantifying the components of a mixture by exploiting their interaction with a stationary phase and a liquid mobile phase under high pressure.
Mobile and stationary phases
The mobile phase is a liquid solvent or a mixture of solvents that carries the sample through the system. The stationary phase is typically a column packed with small, porous particles (often silica-based) that interact differently with each compound.
As the mobile phase flows under high pressure through the stationary phase, each component in the sample moves at a different rate depending on its chemical characteristics and interaction with the column material.
Retention time and elution
Every compound elutes from the column at a different retention time. This is a key indicator used to identify and quantify substances. The sharper and more distinct the elution peaks, the more effective the separation.
Types of HPLC
HPLC can be tailored to different applications through various modes:
- Isocratic Elution: A constant mobile phase composition throughout the run.
- Gradient Elution: Varies the composition of the mobile phase to improve separation of complex mixtures.
- Reverse-Phase HPLC (RP-HPLC): The most common form, using a nonpolar stationary phase and polar mobile phase.
- Normal-Phase HPLC, Ion-Exchange HPLC, and Size-Exclusion HPLC are also used based on the molecular properties of the analytes.
Key components of an HPLC system
Understanding each part of the system is essential for appreciating its versatility and precision, as well as to help understanding how it can benefit from the analytical potential of this technique.
1. Solvent delivery system (Pump)
The pump delivers the mobile phase through the column at a precise and constant flow rate, often between 0.5 to 1.5 mL/min, under pressures of up to 6000 psi.
2. Injector and sample introduction
The injector introduces the sample into the mobile phase. Manual or autosampler injectors are used depending on the system’s automation level. This provides the column with a mixture of the sample and the mobile phase.
3. Column: The Heart of HPLC
This is where the separation happens. Columns vary in length, diameter, and particle size depending on the application. Different types of columns can be used depending on the nature of the sample. Reverse-phase C18 columns are the most widely used in pharmaceutical and biotech labs.
4. Detectors: UV vs Mass Spectrometry (MS)
Traditional systems use UV-Vis detectors to measure absorbance. However, newer systems incorporate Mass Spectrometry (LC-MS) for enhanced specificity. Mass detectors can identify compounds based on molecular weight, offering far superior sensitivity and selectivity.
HPLC vs LC-MS: Enhanced analytical power
Combining HPLC with Mass Spectrometry (LC-MS) brings unmatched analytical power, especially when dealing with complex biological samples. This is thanks to their superior analytical capabilities compared to traditional detection approaches.
The role of LC/MSD iQ integration
At NANBIOSIS Unit 2 (CAbS), researchers have integrated the Agilent G6160A LC/MSD iQ mass selective detector with the Agilent 1260 HPLC system to significantly enhance immunoreagent analysis.
This configuration enables:
- Specific molecular mass detection
- Rapid confirmation of compound identity
- Higher selectivity than UV detectors, even for overlapping peaks
- Improved purification protocols
Advantages over traditional UV detection
Traditional UV detectors may struggle with closely eluting or co-eluting compounds, especially in bioanalytical samples. LC-MS eliminates this by providing a mass fingerprint for each analyte, ensuring better resolution and reducing false positives.
Real Case: Immunoreagent characterization
NANBIOSIS experts at Unit 2 (Custom Antibody Service) use LC/MSD iQ for:
- Monitoring the purity of antibodies
- Quantifying specific immunoreagents
- Characterizing molecular forms for regulatory compliance
This setup supports biotech development pipelines and technology transfer from lab to industry, highlighting the practical utility of this cutting-edge analytical technique.
Applications of HPLC in science and the industry
HPLC is essential across multiple fields where chemical precision is non-negotiable. A few examples are listed herein.
Pharmaceutical Analysis and Quality Control
- Identification and quantification of active pharmaceutical ingredients (APIs)
- Stability testing and degradation analysis
- Regulatory compliance (FDA, EMA)
Biotechnology and Biologics Purification
- Purification of monoclonal antibodies, peptides, and recombinant proteins
- Analytical development for biosimilars and biobetters
- Batch release testing in biomanufacturing
Environmental and Food Safety Testing
- Detection of contaminants, pesticides, or drug residues
- Analysis of food additives, vitamins, and preservatives
- Monitoring of water quality and industrial effluents
Advantages and limitations of HPLC
Strengths
- High precision and reproducibility
- Exceptional resolution of complex mixtures
- Compatibility with a wide range of detectors and samples
- Scalable from analytical to preparative scales
Limitations
- High equipment and maintenance cost
- Requirement of trained personnel
- Complex method development
- Solvent usage and disposal issues
These limitations, however, are mitigated in advanced laboratories through automation, SOPs, and proper training protocols. This is where NANBIOSIS Unit 2 can help you overcome these hurdles.
Future of HPLC: Innovation and automation
There are a few ground-breaking technologies that can potentially revolutionize many analyticial techniques, and HPLC is no exception. The integration of AI, robotics, and cloud-based systems is redefining what HPLC can do.
AI-integrated HPLC platforms
Future systems could incorporate real-time predictive analytics, optimizing flow rates, gradients, and detection settings for maximum efficiency.
Large-scale applications and process control
With sufficient investment, platforms like Agilent LC/MSD iQ can be scaled for industrial-level purification, maintaining accuracy without manual intervention.
Vision from CAbS: NANBIOSIS Expertise
In a scenario with unlimited funding, the team at NANBIOSIS Unit 2 envisions:
- A fully integrated LC/MS-AI platform
- Real-time monitoring and adaptive process control
- Seamless tech transfer from lab to industrial production
- Global-scale immunoreagent production with full traceability
This would elevate the role of HPLC from an analytical tool to a core component of industrial bioprocessing infrastructure.
Conclusion: Why HPLC remains indispensable in Analytical Chemistry
Despite the emergence of newer techniques, HPLC remains the gold standard for separation science. Its adaptability —especially when combined with mass spectrometry— ensures its place in the future of biotech, pharma, and beyond.
Whether you’re developing life-saving biologics, ensuring water safety, or refining analytical workflows, HPLC continues to deliver unmatched resolution, reliability, and reproducibility.
Credits:
Nuria Pascual
Gabriel Alfranca
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









