Researchers use a bacterial toxin domain to boost drug delivery across skin barriers, opening new paths for non-invasive protein-based therapies.
Barcelona, april 2025. Researchers from NANBIOSIS Units at UAB and IR Sant Pau develop innovative strategy for transdermal protein drug delivery using recombinant proteins.
In a major advance in drug delivery research, scientists from NANBIOSIS Unit 1 and Unit 18 at the Universitat Autònoma de Barcelona (UAB) and the Institut de Recerca Sant Pau, affiliated with the CIBER-BBN network, have demonstrated a novel method to increase the permeability of epithelial tissues. By harnessing the non-toxic functional domain of a bacterial toxin, the team has opened new pathways for transdermal and transmucosal delivery of protein-based therapeutics.
The study, recently published in the prestigious journal Molecular Pharmaceutics (American Chemical Society, ACS), was led by Dr. Antonio Villaverde and Dr. Esther Vázquez, with significant contributions from Eric Volta-Durán and other members of NANBIOSIS, such as Ramón Mangues and Isolda Casanova. The research is part of the project PID2020-116174RB-I00, funded by the Agencia Estatal de Investigación (AEI).
Our findings pave the way for the use of recombinant proteins as vehicles in non-invasive transdermal therapies
—Dr. Antonio Villaverde
“Our findings pave the way for the use of recombinant proteins as vehicles in non-invasive transdermal therapies,” explains Dr. Villaverde. “The use of functional domains like c-CPE, derived from natural proteins, shows the power of biotechnology to transform nature’s molecular tools into biomedical innovations.”
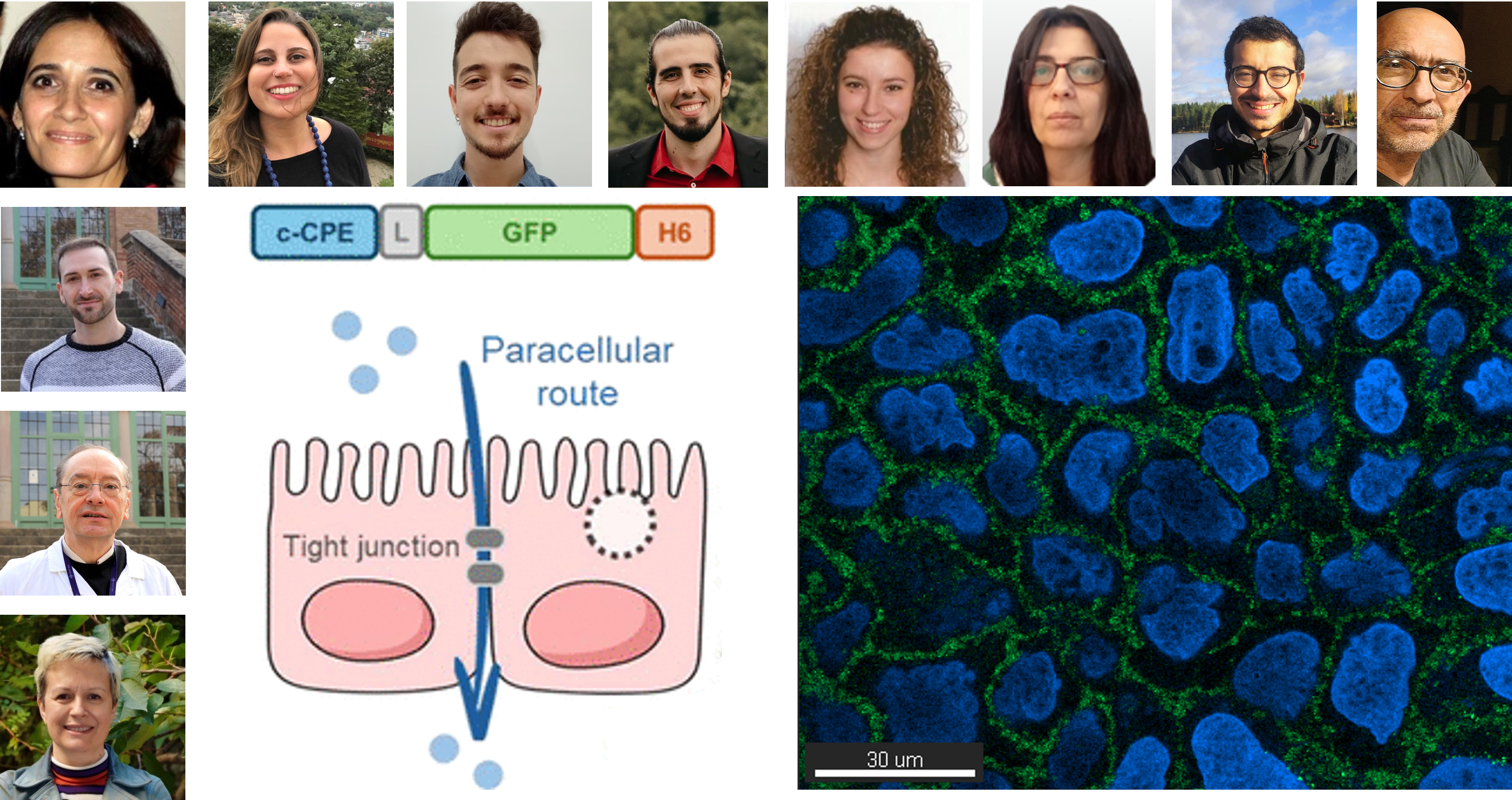
c-CPE: From bacterial toxin to drug delivery enabler
The team focused on c-CPE, a functional domain of the Clostridium perfringens enterotoxin, known for its ability to bind tight junctions between epithelial cells. Through advanced protein engineering and characterization techniques, researchers developed highly pure and stable recombinant proteins containing this domain.
(…) c-CPE enables the passage of other proteins across epithelial monolayers, without inducing toxicity.
—Eric Volta-Durán
“Thanks to our expertise in recombinant protein design and purification, we created multifunctional proteins that efficiently bind to cell-cell contact regions,” says Eric Volta-Durán. “Using confocal microscopy, we confirmed that c-CPE enables the passage of other proteins across epithelial monolayers, without inducing toxicity.”
Interestingly, the study found that while c-CPE-fused proteins strongly attach to tight junctions and do not themselves cross the barrier, they act as trans-permeabilization agents—allowing other, non-modified proteins to pass through epithelial layers.
This paradoxical “trans-mediated, cis-inhibited” behavior of c-CPE suggests a promising mechanism for enhancing drug bioavailability in non-invasive therapeutic strategies, including topical applications, wound healing, and transmucosal treatments.
Cutting-edge nanobiotechnology for precision medicine
This breakthrough is a prime example of how NANBIOSIS supports the development of frontier biotechnological tools. The research benefited from the advanced capabilities of NANBIOSIS Unit 1 and Unit 18, located at UAB and IR Sant Pau respectivelly, including facilities for protein production, purification, characterization, and nanomedicine testing.
By converting a natural bacterial toxin into a helpful ally, the study highlights the transformative potential of biotechnology and nanomedicine for improving therapeutic delivery across complex biological barriers.
Reference:
Sanchez JM, Favaro MTP, López-Laguna H, Parladé E, Di Somma A, Casanova I, Unzueta U, Mangues R, Vázquez E, Voltà-Durán E, Villaverde A. Trans-Mediated, Cis-Inhibited Paradoxal Activity of Clostridium perfringens Enterotoxin (c-CPE) in Modulating Epithelial Permeability. Molecular Pharmaceutics. https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.4c01205

What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









