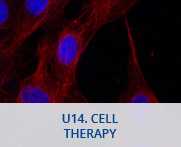
U14. Cell Therapy
Laboratory with an area of 81,02 m2 for cell biology and molecular biology techniques. The laboratory is also equipped with two cell culture rooms for the isolation, expansion and characterization of different cell types. FOR THOSE SERVICES IDENTIFIED AS OUTSTANDING, AT LEAST 20% OF THEIR CAPACITY IS OPEN UNDER COMPETITIVE ACCESS. SEE ANNEX 1 OF ACCESS PROTOCOL FOR DETAILS ON % OF OPENNESS FOR EACH SERVICE


U14. Cell Therapy
Description
Services
Active projects