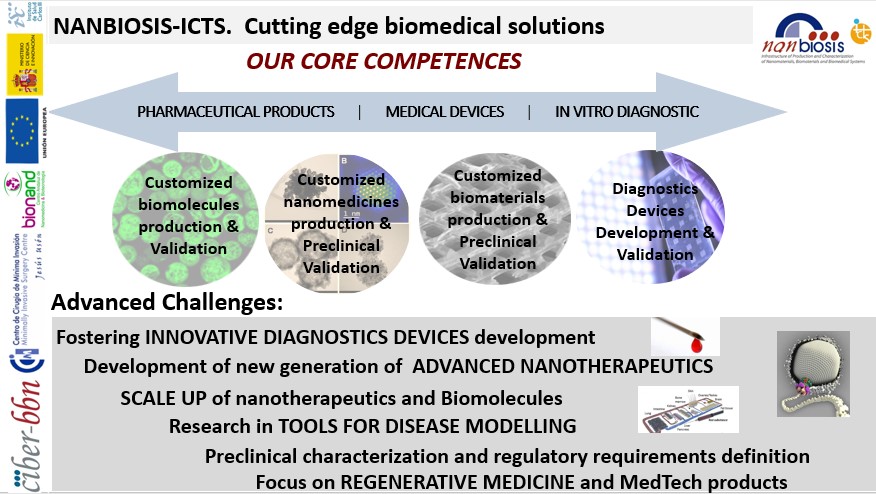
New NANBIOSIS’ focus on Cutting-Edge Biomedical Solutions
We are delighted to announce the publication of our new corporate brochure which reflects NANBIOSIS’s main core competences. This fresh looking promotion material has been intentionally designed to emphasize our experience of join expertise and capabilities solving problems in biomedical research, focussing on quality, adaptability and excellence commitment.
The NANBIOSIS’ Cutting-Edge biomedical solutions have been updated to offer a wider range of Integrated solutions to advanced challenges faced by biomedical researchers in the fields of tissue engineering, regenerative medicine, diagnostic and medical devices.
The Cutting-Edge biomedical solutions offered by the ICTS NANBIOSIS have been organised drilling down on our key areas:
- Customized biomolecules production & Validation
- Customized nanomedicines production & Preclinical Validation
- Customized biomaterials production & Preclinical Validation
- Diagnostic Devices production & Validation