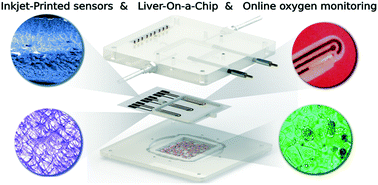
Online oxygen monitoring using integrated inkjetprinted sensors in a liver-on-a-chip system
Scientists of NANBIOSIS Unit 8. Micro – Nano Technology Unit, led by Gemma Gabriel, Scientific Coordinator of the Unit, are the authors of the article “Online oxygen monitoring using integrated inkjetprinted sensors in a liver-on-a-chip system”, published by Lab on a chip.
The demand for real-time monitoring of cell functions and cell conditions has dramatically increased with the emergence of organ-on-a-chip (OOC) systems. However, the incorporation of co-cultures and microfluidic channels in OOC systems increases their biological complexity and therefore makes the analysis and monitoring of analytical parameters inside the device more difficult. In this work, theauthors present an approach to integrate multiple sensors in an extremely thin, porous and delicate membrane inside a liver-on-a-chip device. Specifically, three electrochemical dissolved oxygen (DO) sensors were inkjet-printed along the microfluidic channel allowing local online monitoring of oxygen concentrations. This approach demonstrates the existence of an oxygen gradient up to 17.5% for rat hepatocytes and 32.5% for human hepatocytes along the bottom channel. Such gradients are considered crucial for the appearance of zonation of the liver. Inkjet printing (IJP) was the selected technology as it allows drop on demand material deposition compatible with delicate substrates, as used in this study, which cannot withstand temperatures higher than 130 °C. For the deposition of uniform gold and silver conductive inks on the porous membrane, a primer layer using SU-8 dielectric material was used to seal the porosity of the membrane at defined areas, with the aim of building a uniform sensor device. As a proof-of-concept, experiments with cell cultures of primary human and rat hepatocytes were performed, and oxygen consumption rate was stimulated with carbonyl-cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), accelerating the basal respiration of 0.23 ± 0.07 nmol s−1/106 cells up to 5.95 ± 0.67 nmol s−1/106 cells s for rat cells and the basal respiration of 0.17 ± 0.10 nmol s−1/106 cells by up to 10.62 ± 1.15 nmol s−1/106 cells for human cells, with higher oxygen consumption of the cells seeded at the outflow zone. These results demonstrate that the approach of printing sensors inside an OOC has tremendous potential because IJP is a feasible technique for the integration of different sensors for evaluating metabolic activity of cells, and overcomes one of the major challenges still remaining on how to tap the full potential of OOC systems.
Article of reference: DOI: 10.1039/C8LC00456K
This article is part of the themed collection: Organ-, body- and disease-on-a-chip systems