Rare diseases international day 2023: some NANBIOSIS contributions
Today is the international day of rare diseases, a day to raise awareness and instigate change for people living with a rare disease. From NANBIOSIS we want to sume to this celebration and higtligh our commitment to helping people with rare diseases through research.
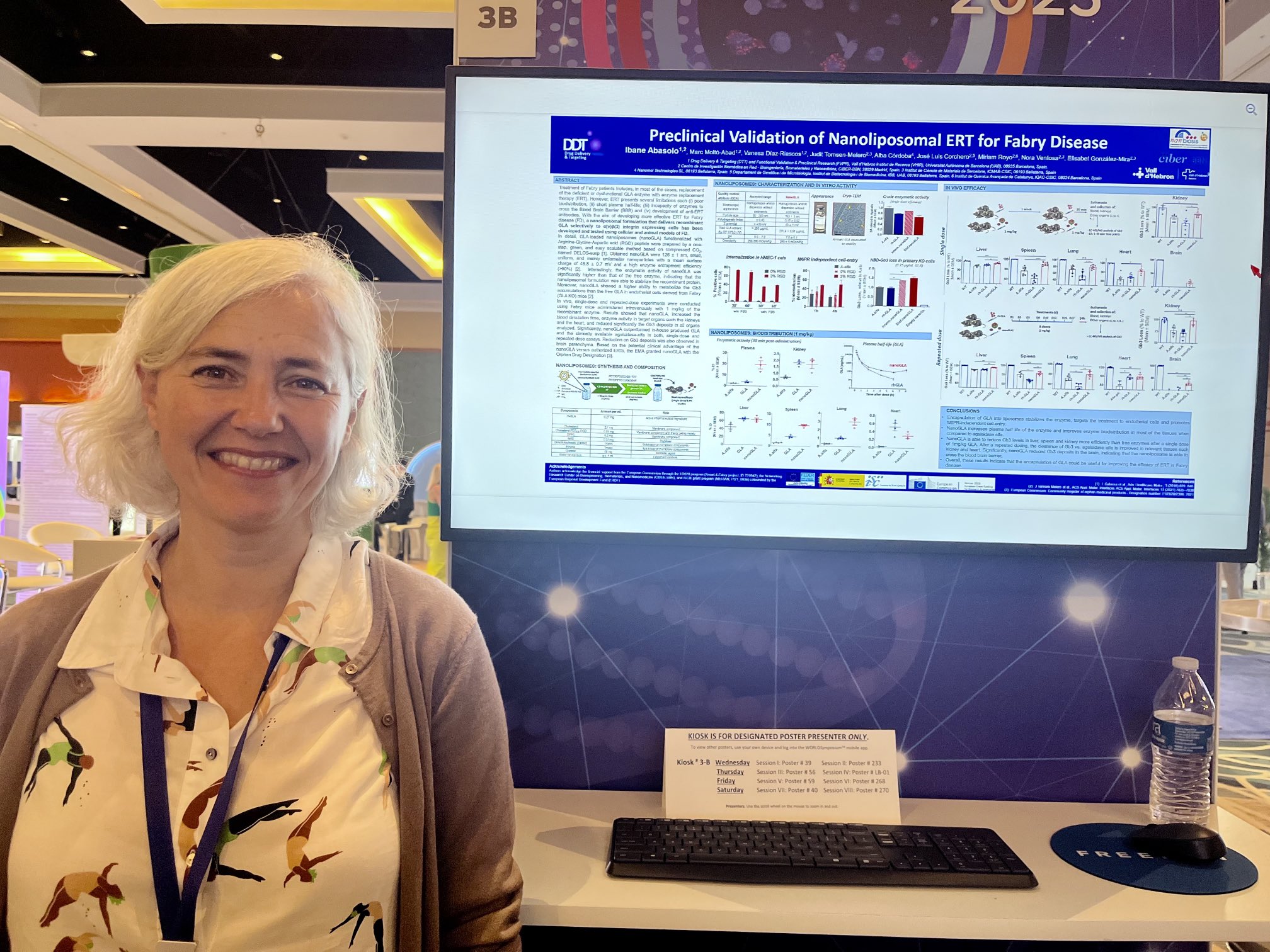
Dr. Ibane Abasolo, Scientific Director of NANBIOSIS U20, was at the WORLDSymposia conference last week in Orlando (FL, USA), where the latest advances in preclinical study and clinic of lysosomal storage diseases were explained. There, she presented the results obtained in the Smart4Fabry project coordinated by the CIBER-BBN where nanoliposomes were developed for the treatment of Fabry disease. The work, entitled “Preclinical Validation of Nanoliposomes for ERT for Fabry disease”, was a result of the collaboration of the groups of Dr. Ventosa and Dr. Corchero, both from CIBER-BBN, and the participation of units U1, U3, U6, and U20 of the ICTS Nanbiosis.

In addition, today Dr. Abasolo participated in the Nano Rare Day session, organized by the NanoMedSpain platform and the Barcelona Bioengineering Institute (IBEC) at the Sant Joan de Deu Hospital in Barcelona, presented the work entitled “Use of natural and artificial nanoparticles for the treatment of lysosomal storage diseases”, where in addition to nanoliposomes, she also detailed how extracellular vesicles can be a good vehicle to improve replacement enzyme therapy in lysosomal diseases.
Also Dr. Juan Pablo Salvador from NANBIOSIS U2 CAbS has presented at in the Nano Rare Day session his talk on “Quorum Sensing to improve the management of cysticfibrosis“, explaining the difficulty of quickly identifying bacterial infections, which are common in patients with Cystic Fibrosis. In this sense, “Quorum Sensing”, a microbial communication mechanism through which the cells themselves regulate the expression of genes based on cell density, can help identify biomarkers and improve the management of cystic fibrosis.

Related news: Fabry Desease in the Rare Disease Day: A New Hope