Gold nanoparticles can activate drugs inside tumors
Jesus Santamaría, Scientific Director of Unit 9 of NANBIOSIS has participated in a study that shows the ability of gold nanoparticles to generate in situ potent anticancer drugs from inert molecules thanks to a mechanism of elimination of terminal chemical groups that nanometric gold is able to catalyze. Gold is ideal for this catalytic role due to its high biocompatibility.
These results, published in the journal Angewandte Chemie, offer new hope in the fight against cancer and have been obtained thanks to the collaboration of scientists of Unit 9 of NANBIOSIS and, Víctor Sebastián, Silvia Irusta and Jesús Santamaría, with researchers from the Center for Cancer Research at the University of Edinburgh, led by Dr. Unciti-Broceta.
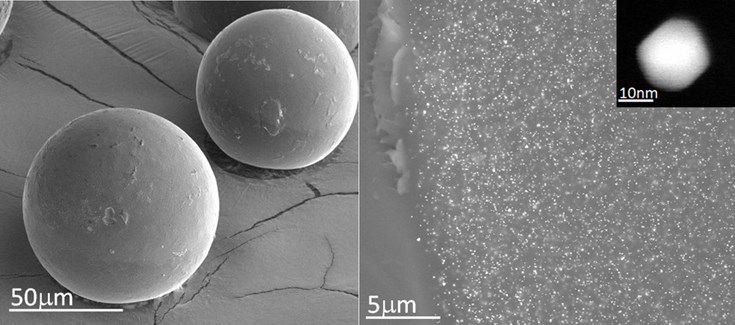
The work reveals the possibility of carrying out catalysis in biological means using tiny particles of gold. These gold nanoparticles, camouflaged in a resin microcapsule implanted in the brain of a zebrafish, have succeeded in catalyzing a chemical reaction generating fluorescent compounds.
Significant practical importance
“The main problem of chemotherapy treatments are the side effects in various organs due to the toxicity of the molecules that are used to fight cancer. For this reason, alternative routes are explored from nanotechnology, for example, transporting drugs to the tumor using nanoparticles or alternative treatments to drugs, such as hyperthermia, elevation of local temperature, obtained with nanoparticles”, says Jesús Santamaría.
The conclusions of this work suggest a different way: the drug would be supplied to the patient in its inert form and only converted to the toxic form locally, thanks to the catalysis of the nanoparticles that a surgeon would implant in the tumor.
Article of reference
Pérez-López, A. M., Rubio-Ruiz, B., Sebastián, V., Hamilton, L., Adam, C., Bray, T. L., Irusta, S., Brennan, P. M., Lloyd-Jones, G. C., Sieger, D., Santamaría, J. and Unciti-Broceta, A. (2017). “Gold-Triggered Uncaging Chemistry in Living Systems”. Angew. Chem. Int. Ed.. doi:10.1002/anie.201708379