Rare diseases Day February 29: combating Fabry Disease
29 of February is a ‘rare’ date and February, a month with a ‘rare’ number of days, has become a month to raise awareness about rare diseases and their impact on patients’ lives. Since 2008 thousands of events happen every year all around the world and around the last day of February.
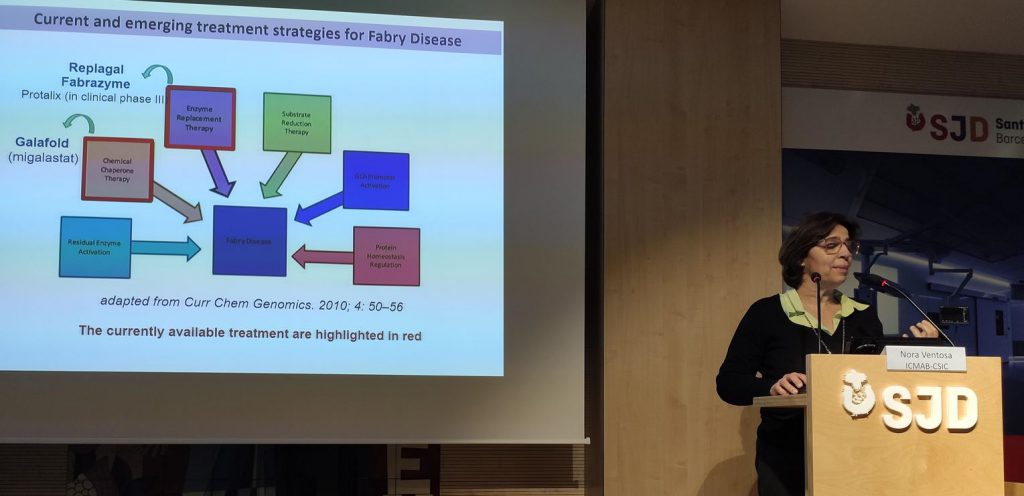
NanoMed Spain Platform and the Hospital of Sant Joan de Déu have organized the NanoRareDiseaseDay to present the latest innovations in the field of Nanomedicine for the treatment and diagnosis of rare diseases (diseases affecting less than 5 people per 10,000 inhabitants). Nora Ventosa, Scientific Director of NANBIOSIS U6 Biomaterial Processing and Nanostructuring Unit (CIBER-BBN / ICMAB-CSIC) presented Smart4Fabry a European project with the aim of reducing the Fabry disease treatment cost and improve the life-quality of Fabry disease patients
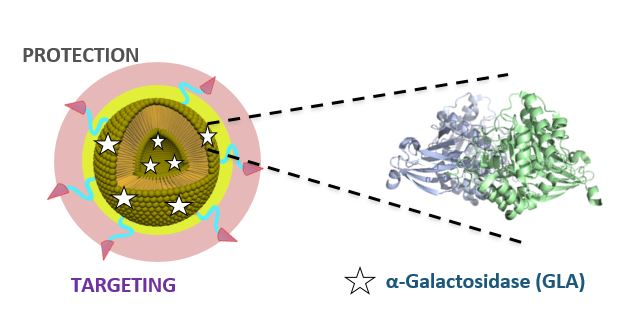
Fabry disease is one of the rare diseases that currently lack a definitive cure. It is cause by lysosomal storage disorders (LSDs): the deficiency of α-Galactosidase A (GLA) enzyme activity result in the cellular accumulation of neutral glycosphingolipids, leading to widespread vasculopathy with particular detriment to the kidneys, heart and central nervous system.
Smart-4-Fabry has been conceived to obtain a new nanoformulation of GLA, that will improve the efficacy and toleration compared to the actual treatment with non-formulated GLA. Four units of NANBIOSIS participate in the project:
– U1 Protein Production Platform (PPP) led by Neus Ferrer and Antony Villaverde at IBB-UAB accomplish the production and purification in different expression systems for R&D purposes.
– U3 Synthesis of Peptides Unit led by Miriam Royo at IQAC-CSIC performs all the chemical process of the Smart-4-Fabry project, i.e. design and synthesis of peptides used as targeting ligands in the nanoliposome formulation
– U6 Biomaterial Processing and Nanostructuring Unit led by Nora Ventosa and Jaume Veciana at ICMAB-CSIC undertakes tasks related to the manufacture of the nanoliposome formulation of GLA enzyme and the physico-chemical characterization (this unit counts with plants at different scales, from mL to L, which allow process development by QbD and process scale-up, as well as instrumental techniques for assessment of particle size distribution, particle concentration, particle morphology and stability, and Z-potential)
– U20 In Vivo Experimental Platform led by Simó Schwartz and Ibane Abásolo at VHIR to carry out the non-GLP preclinical assays of the project (in vivo efficacy, biodistribution and tolerance/toxicity assays).
For further information about Fabry disease and the Smart4Fabry project: here
project on nanoliposomes development for the treatment of Fabry disease
(Pictures by Nanomed Spain)