New cover ACS Sustainable Che. Eng. by Scientists of NANBOSIS U9
Scientists of NANBIOSIS Unit 9 Synthesis of Nanoparticles Unit are coauthors of of the New cover ACS Sustainable Chem. Eng.
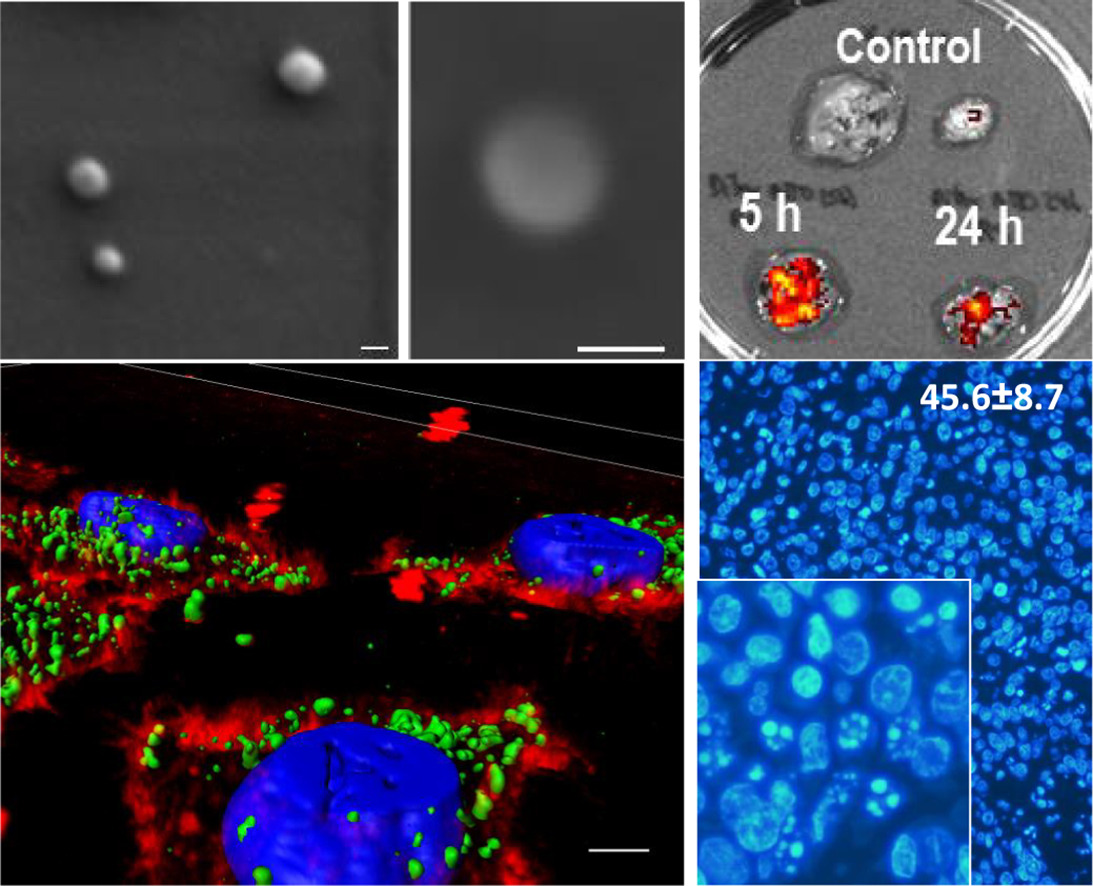
Sustainable Production of Drug-Loaded Particles by Membrane Emulsification. Albisa A, Piacentini E, Arruebo M, Sebastian V, Giorno L. ACS Sustainable Chem. Eng., 2018, 6 (5), pp 6663–6674 March 13, 2018. DOI: 10.1021/acssuschemeng.8b00401. IF: 5,951