A discovery in one of the most aggressive cancers will allow more efficient diagnosis
The extracellular vesicles secreted by triple-negative breast cancer stem cells are markers of lung metastasis, according to a study carried out by researchers at CIBER.
The work has been carried out by researchers from various CIBER-BBN groups (Bioengineering, Biomaterials and Nanomedicia), and CIBERONC (CIBER area focused on cancer) has participated in it. The research has been led by Joaquín Seras, from the Vall d’Hebron Research Institute (VHIR), a specialist in targeted drug therapies.
Physicochemical EVs characterization and all the in vivo studies were performed by NANBIOSISunits of CIBER, specifically NTA analysis was carried out at Unit 6 of Biomaterial Processing and Nanostructuring, led by Nora Ventosa at ICMB-CSIC and animal experimentation at Unit 20 “In vivo experimental platform”, led by Ibane Abasolo at VHIR.
The vesicle, in cell biology, is an organelle that forms a small, closed compartment, separated from the cytoplasm by a lipid bilayer just like the cell membrane. The vesicles store, transport or digest cellular products and waste. According to Joaquin Seras, leader of the research: “the identification of this subpopulation of cancerous extracellular vesicles, and their important role in the progression of the disease, will allow in the future to develop systems more effective and less invasive diagnostic methods based on their detection directly from blood samples”.
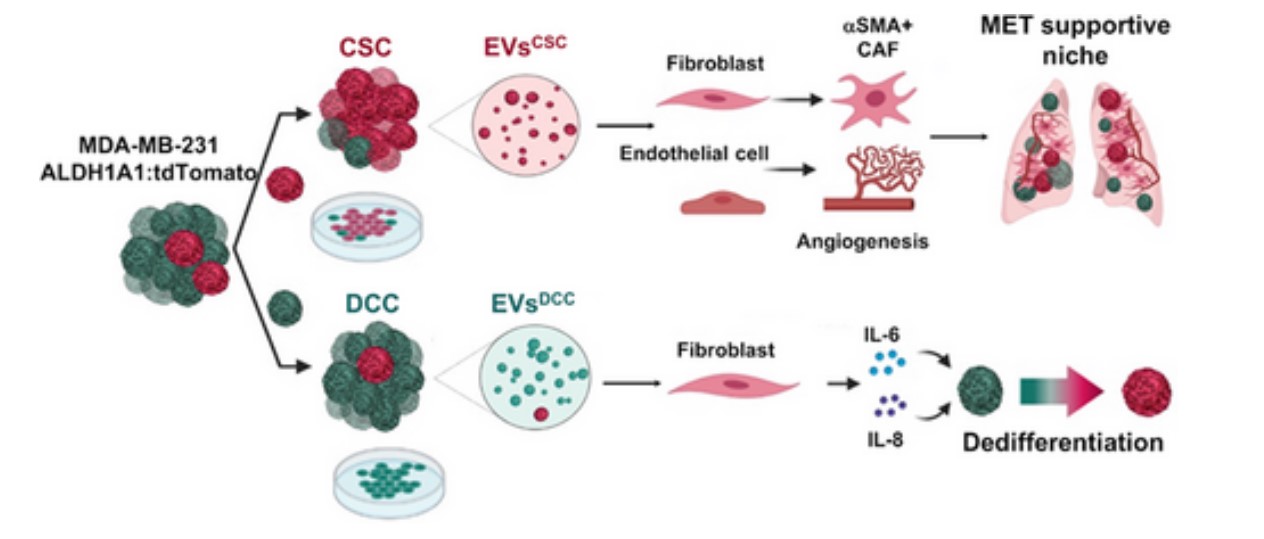
In different types of tumors, including triple negative breast cancer, it has been observed that the extracellular vesicles generated by tumor cells play an important role in the generation of pre-metastatic niches. Triple negative breast cancer, one of the most aggressive, highly plastic and heterogeneous, is characterized by a significant presence of malignant stem cells.
The study carried out by the Spanish researchers from CIBER with promising results, published in the “International Journal of Cancer”, shows, both in in vitro and in vivo models of the disease, that the vesicles actively contribute to the formation of areas with favorable conditions for the formation of metastases, thus favoring way, the spread of the disease.
Research contributions
In the opinion of Joaquin Seras, the great contribution of this work is that it “describes how the extracellular vesicles secreted by certain subpopulations of cancer cells, specifically those derived from cancer stem cells, have the potential to modify the microenvironment of the future metastatic niche to promote tumor growth.
In other words, continues the leader of the study: “the research sheds new information on the pathogenic mechanism of the disease, and suggests these extracellular vesicles as markers with diagnostic potential. It should be noted that these nanoparticles are secreted into the bloodstream by tumor cells, and effective capture and identification would allow them to be exploited as a diagnostic tool”.
On the characterization of extracellular vesicles of cancer cells
The complex composition and functional differentiation of cancer cells in a tumor also increases the heterogeneity of the subsets of vesicles secreted by cancer.
This phenomenon is particularly relevant in triple negative breast cancer, one of the most aggressive, highly plastic and heterogeneous cancers, characterized by a significant presence of malignant stem cells. However, until now the diversity of the vesicles secreted by cancer cells had not been studied, a diversity that is closely related, in turn and as the study shows, to cellular heterogeneity in triple-negative tumors.
The importance of the CIBER study lies at this point: the vesicles secreted by different tumor subpopulations and grouped by their degree of differentiation show fundamentally different activities in terms of their impact on cancer progression.
In the investigation, the extracellular vesicles secreted by up to three different types of neoplastic cells have been isolated and characterized, observing different bioburdens for each type, with the consequent differential effect on stromal cells. In addition, and as the study shows, cancer stem cell-derived vesicles contribute to converting healthy lung cells into receptive niches for the metastatic growth of cancerous breast cells.
Article reference:
González-Callejo P, Gener P, Díaz-Riascos ZV, Conti S, Cámara-Sánchez P, Riera R, Mancilla S, García-Gabilondo M, Peg V, Arango D, Rosell A, Labernadie A, Trepat X, Albertazzi L, Schwartz S Jr, Seras-Franzoso J, Abasolo I. Extracellular vesicles secreted by triple-negative breast cancer stem cells trigger premetastatic niche remodeling and metastatic growth in the lungs. Int J Cancer. 2023 Jan 27. doi: 10.1002/ijc.34447. Epub ahead of print. PMID: 36705298.