Colloidal phenomena in COVID-19
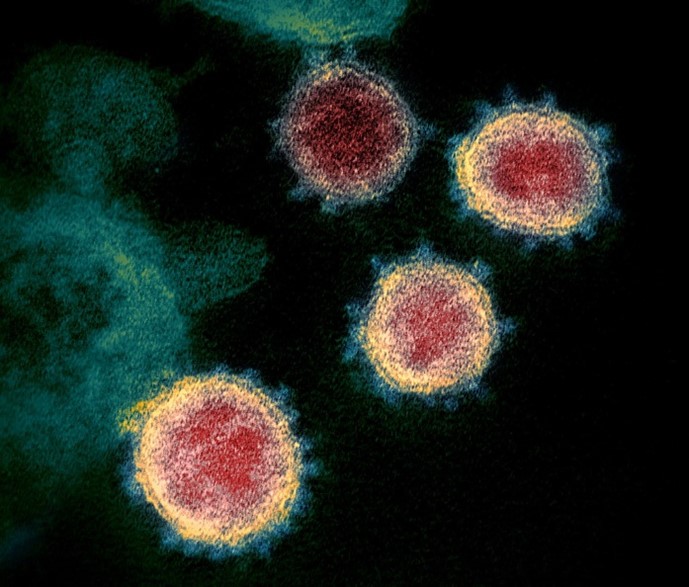
Transmission electron microscope image of SARS-CoV-2 (National Institute of Allergy and Infectious Diseases https://www.niaid.nih.gov)
The special volume (No. 55) of the journal Current Opinion in Colloid and Interface Science reviews the implications of colloidal science in the phenomenology of COVID-19, for which the techniques available in NANBIOSIS U12, “Nanostructured liquid characterization unit” , are relevant.
Two articles to highlight in this special volume:
- Airborne transmission of the virus through droplets, and the effect of evaporation and sedimentation. Airborne transmission is determined by the settling time, that is, the time it takes for droplets to be in the air before settling. Evaporation increases the settling time by reducing the mass of the droplets. In fact, the small droplets can, depending on their solute content, evaporate almost completely and remain in the air for a long time. Considering that viruses possibly remain infectious for hours in the form of aerosols, the formation of droplet nuclei can substantially increase the infectious viral airborne load. The article reviews the physical-chemical factors that control the evaporation and sedimentation times of droplets and play an important role in determining the risk of airborne infection. (https://www.sciencedirect.com/science/article/pii/S1359029421000558)
- The interactions between surfactants and viruses, which act on different components such as the lipid envelope, the membrane proteins (envelope) and the nucleocapsid proteins. Surfactants play very important roles, either directly, as in disinfection, or as carrier components of drug delivery systems for prophylaxis or treatment. By designing tailor-made surfactants and consequently advanced formulations, an increasingly effective use of surfactants can be expected, either directly as antiviral compounds or as part of more complex formulations. (https://www.sciencedirect.com/science/article/pii/S1359029421000637)
In summary, colloid science can contribute in a multidisciplinary strategy to fight pandemics.
By Carlos Rodriguez Abreu, Scientific Director of NANBIOSIS U12