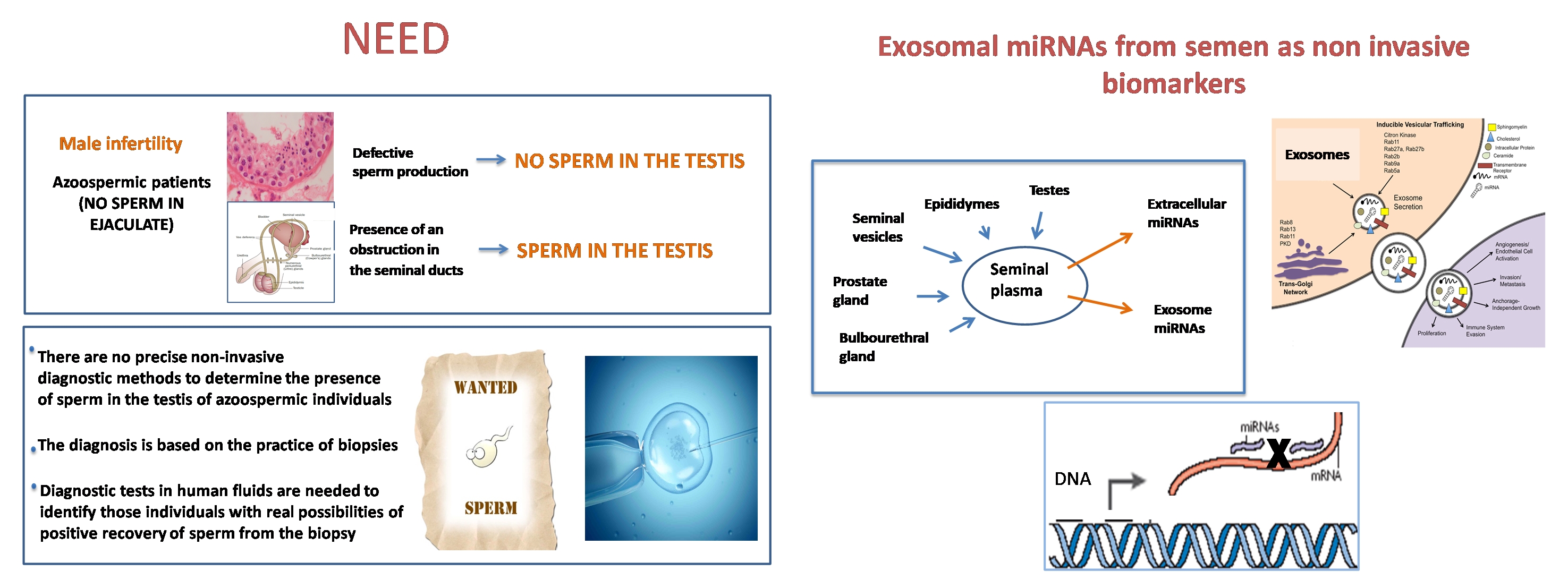
Infertility: Exosomal microRNAs in seminal plasma as sensitive and specific biomarkers of the presence of testicular spermatozoa for azoospermia
NANBIOSIS ICTS coordination equipment has recently received from researchers of Human Molecular Genetics Group of Bellvitge Biomedical Research Institute (IDIBELL) a publication mentioning NANBIOSIS in the Acknowledgements for its participation in the results of its investigations. The article has been publish by the journal Human Reproduction of OXFORD UNIV PRESS with Quartile 1/ Decile 1.
The study carried out by Maria Barcelo, Ana Mata, Lluís Bassas, and Sara Larriba demonstrates the potential of several miRNAs contained in small extracellular vesicles (sEVs) of seminal fluid as sensitive and specific biomarkers for selecting those azoospermic individuals with real chances of obtaining spermatozoa from the testicular biopsy. The nanoparticle tracking analysis was performed by the ICTS NANBIOSIS U6 Biomaterial Processing and Nanostructuring Unit.
Article of reference:
Maria Barceló, Ana Mata, Lluís Bassas and Sara Larriba, Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue Human Reproduction, pp. 1–12, 2018doi:10.1093/humrep/dey072