Laparoendoscopic Single-Site Surgery Using Handheld Robotic Device
Francisco Miguel Sánchez Margallo, Scientific Director of CCMIJU and NANBIOSIS U21. Experimental operating rooms, is co-author of the publication “Assessment of Postural Ergonomics and Surgical Performance in Laparoendoscopic Single-Site Surgery Using a Handheld Robotic Device“, by Surgical Innovation (SAGE journal).
The study results show a positive learning curve in ergonomics and surgical performance using the robotic instrument during LESS surgery. This instrument improves the surgeon’s body posture and the needle positioning errors. The use of the robotic instrument is feasible and safe during LESS partial nephrectomy and sigmoidectomy procedures.
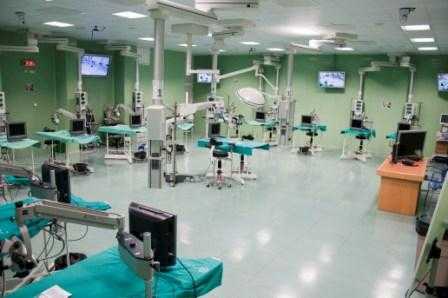
The study has been develloped with surgical facilities of high technology that allow in vivo efficacy assays of drugs, nanomedicines, biomaterials and others, performed at unit 21 of NANBIOSIS.
For further information: DOI: 10.1177/1553350618759768